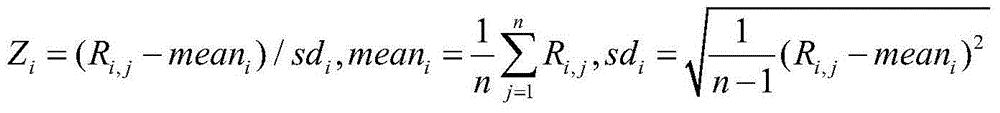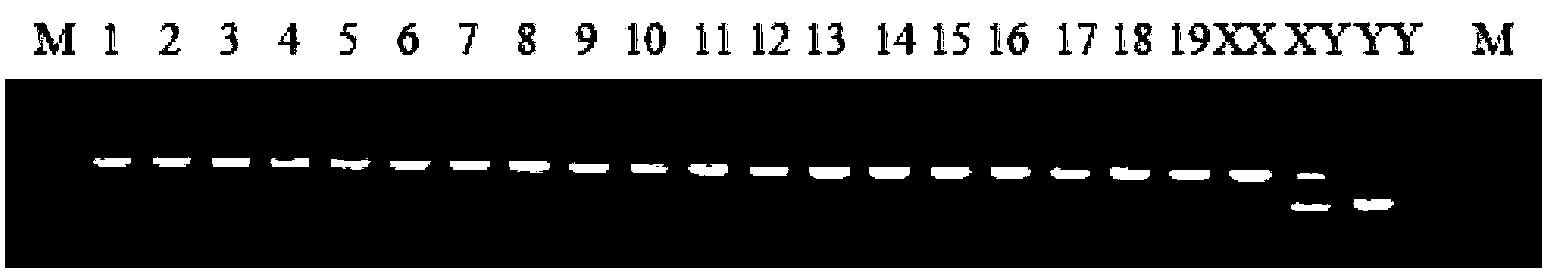Patents
Literature
31 results about "Normal female" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
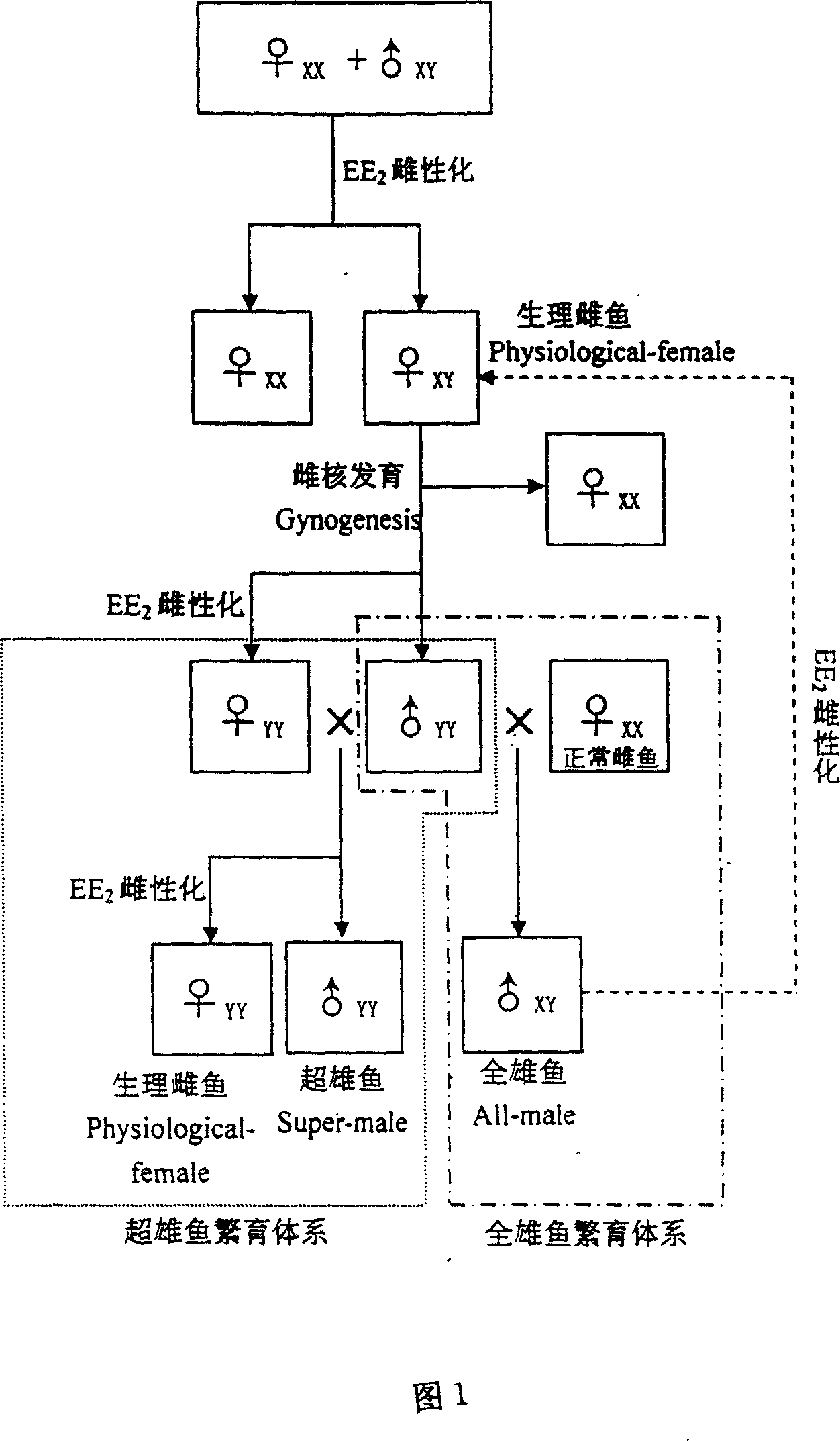
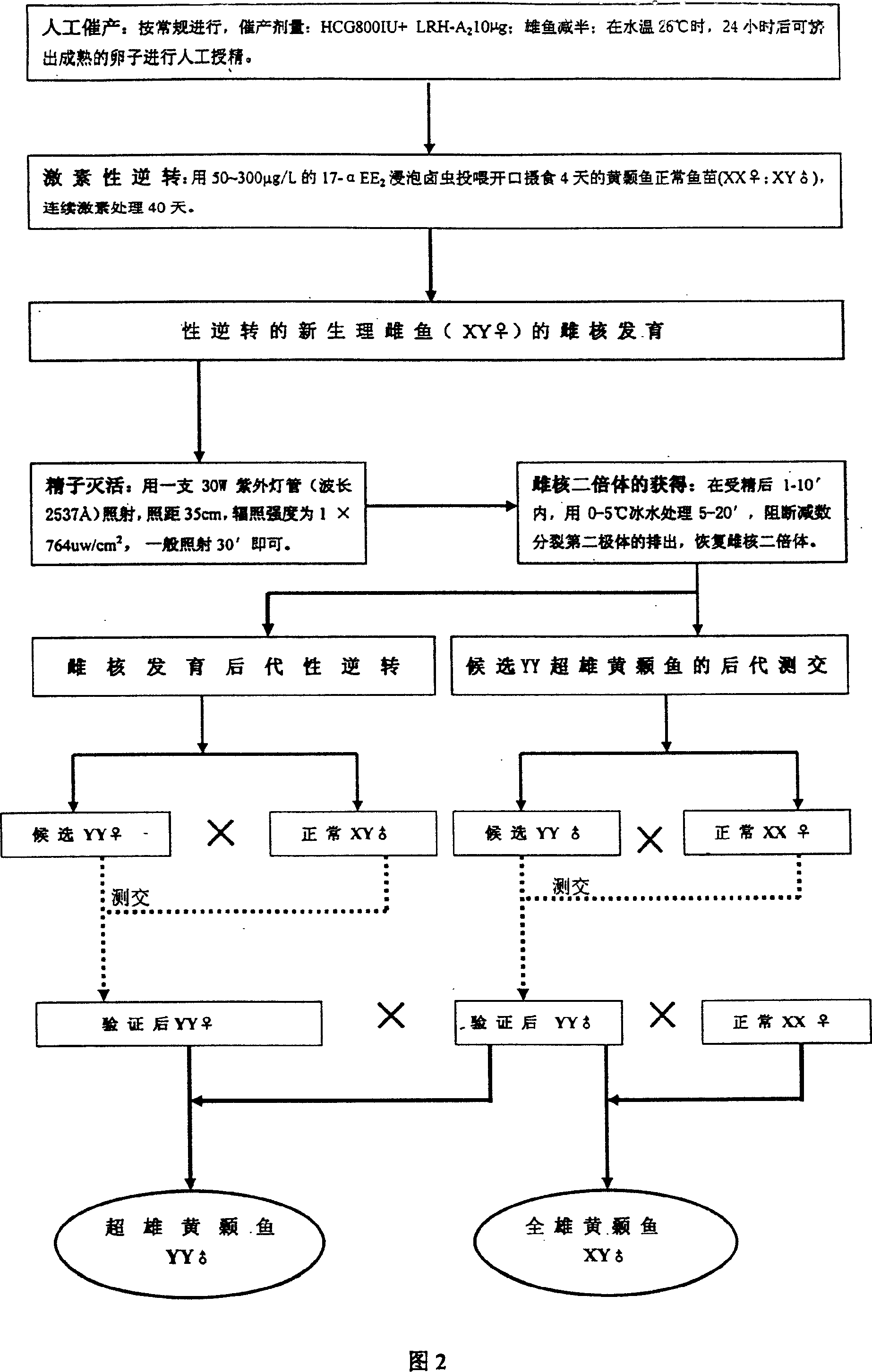
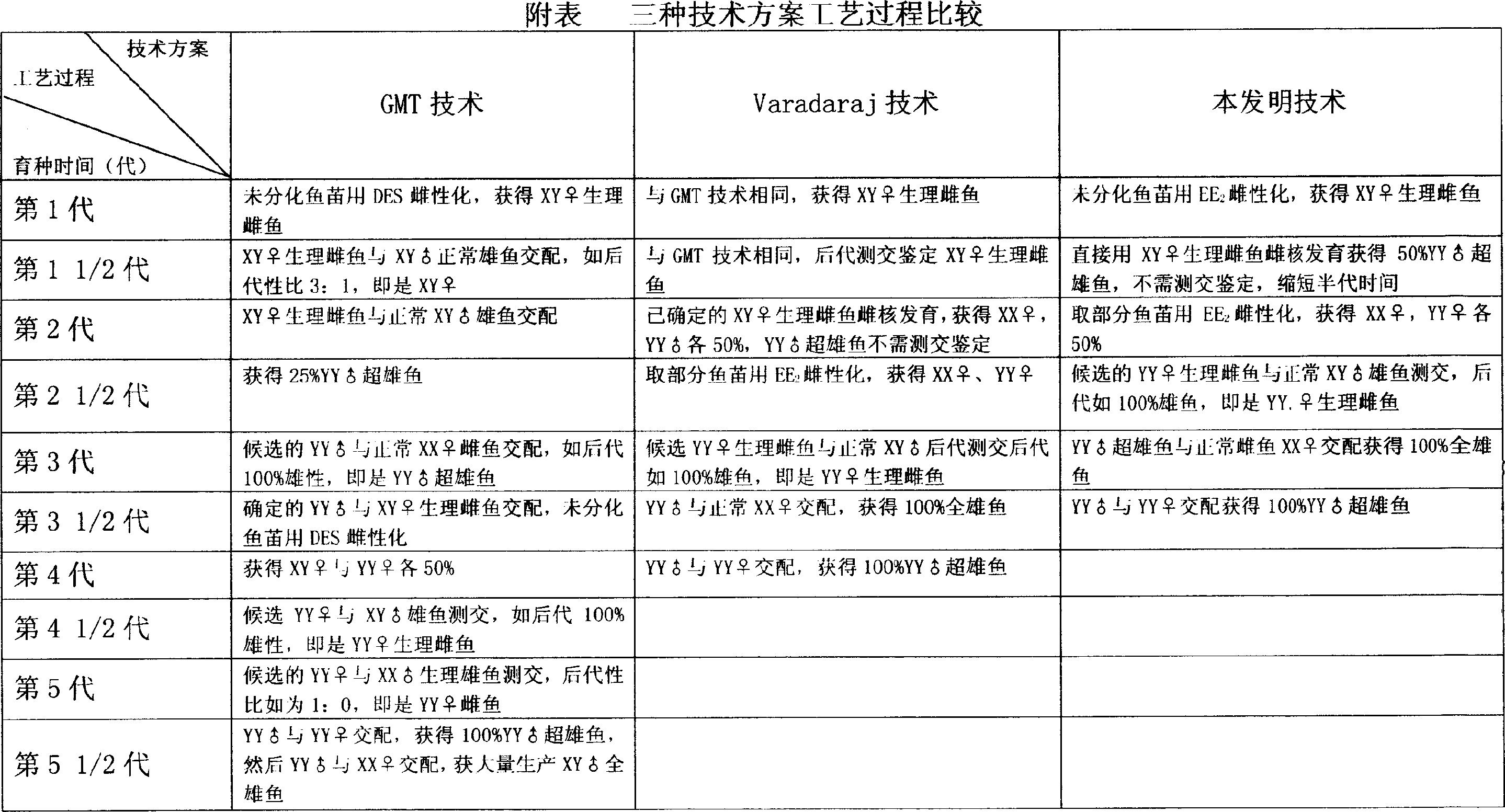
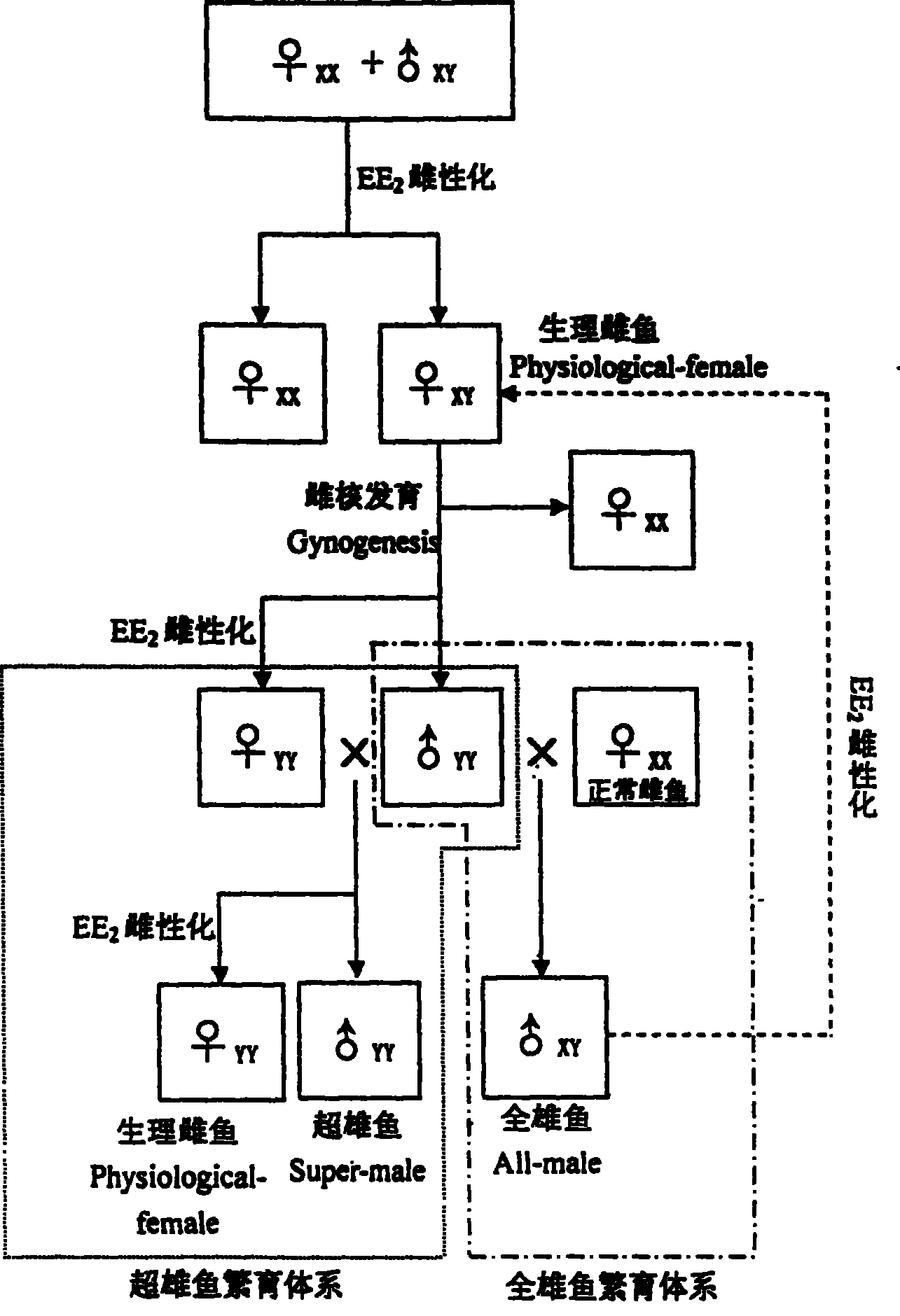
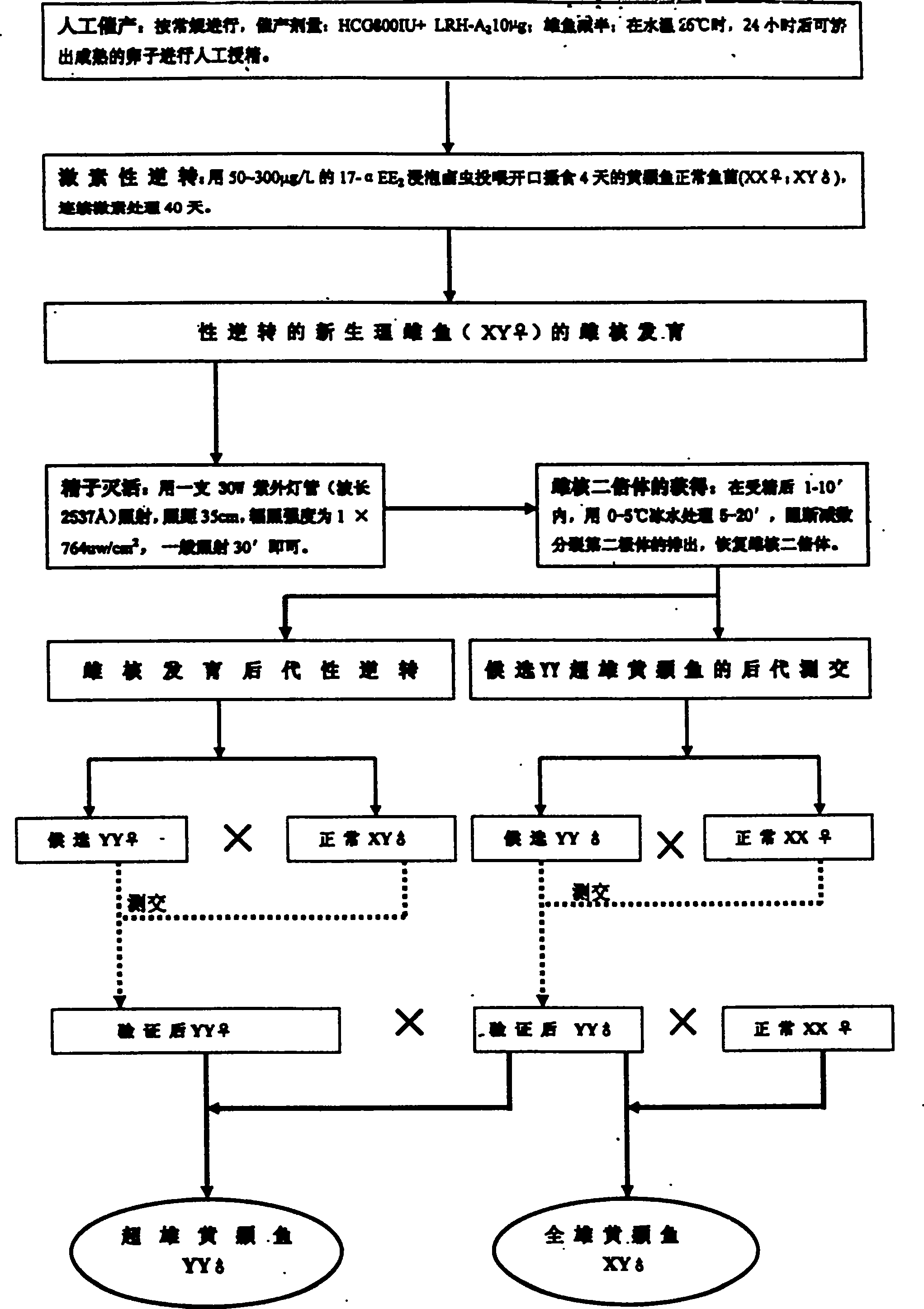
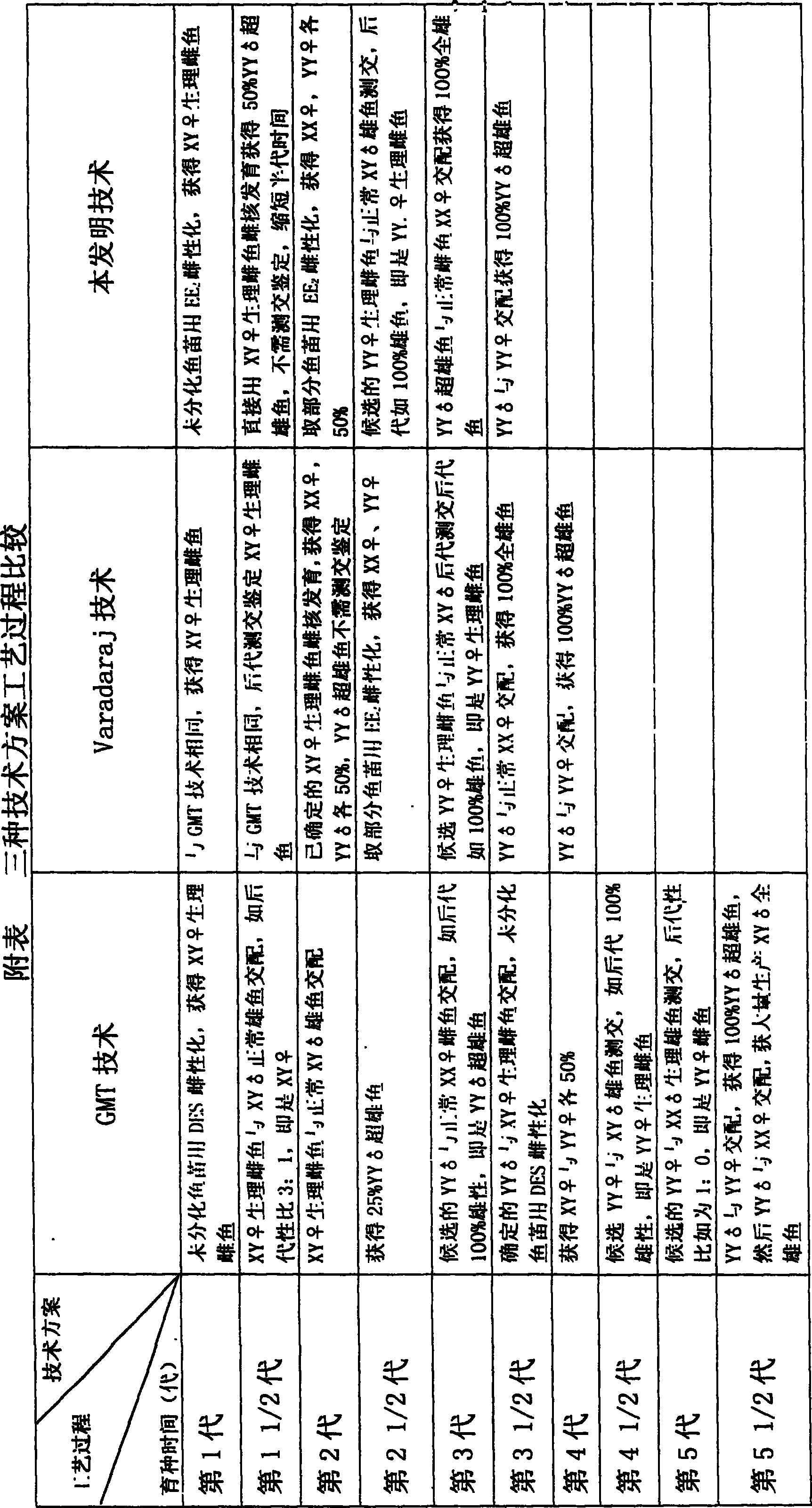
Method for continuously producing complete male pelteobagrus fulvidraco by sex reversion and embryonic development
InactiveCN1994072AShorten breeding timeImprove ergonomicsAnimal reproductionClimate change adaptationNormal femaleFishery
The invention relates to a method for producing male fish, wherein it comprises that: artificially inducing; artificially inducing the female and male fishes; artificial insemination; inverting hormone; feeding seed with Artemia which contains hormone; converting male into biological female; the female grows. The invention comprises sperm inactivation and female diploid generation, to generate ready male fish and normal female fish; crosses the male fish and female fish to obtain the full male fish; inverts the male fish into biological female fish to cross with male fish, to obtain the full super male fish.
Owner:INST OF AQUATIC LIFE ACAD SINICA
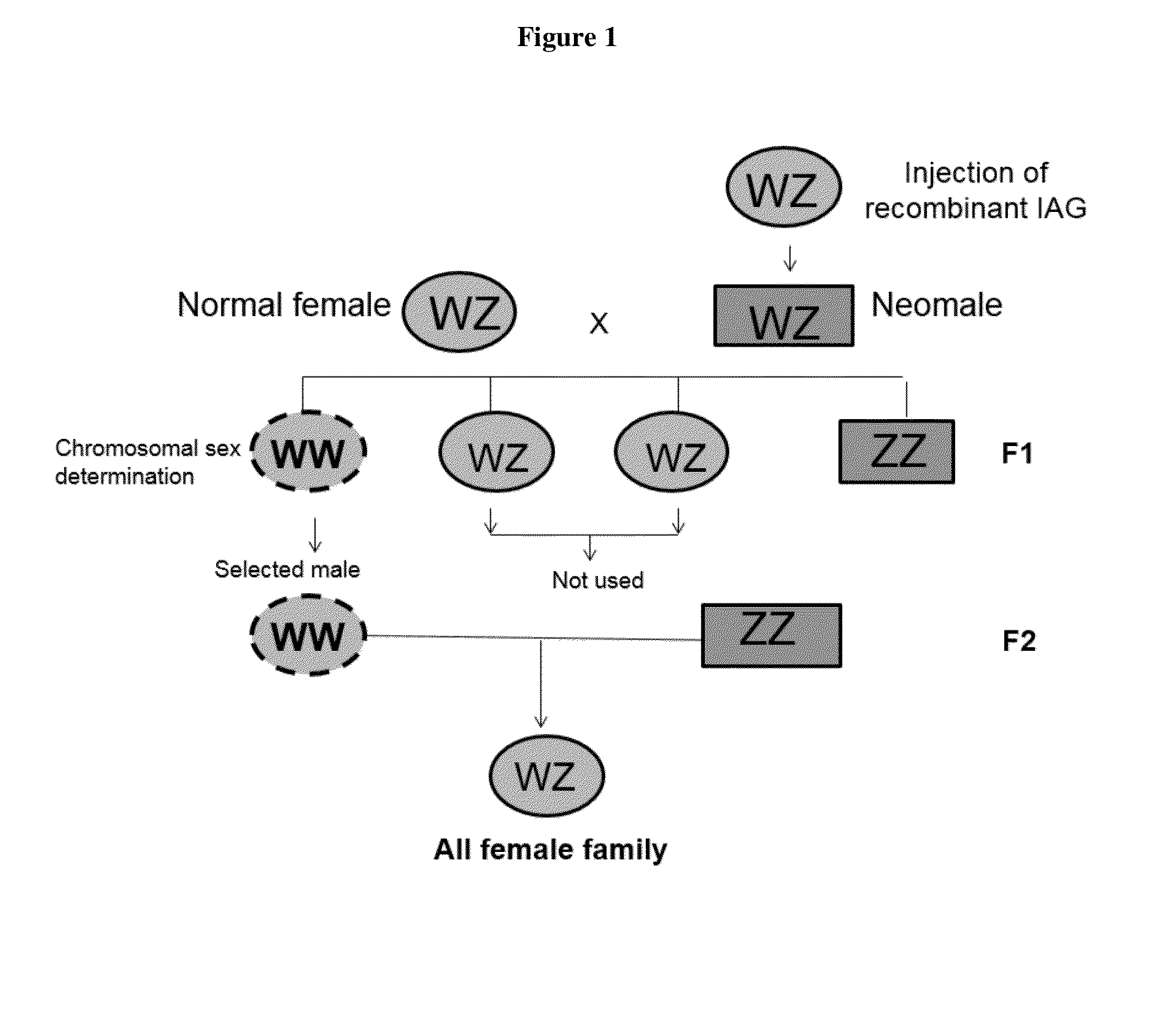
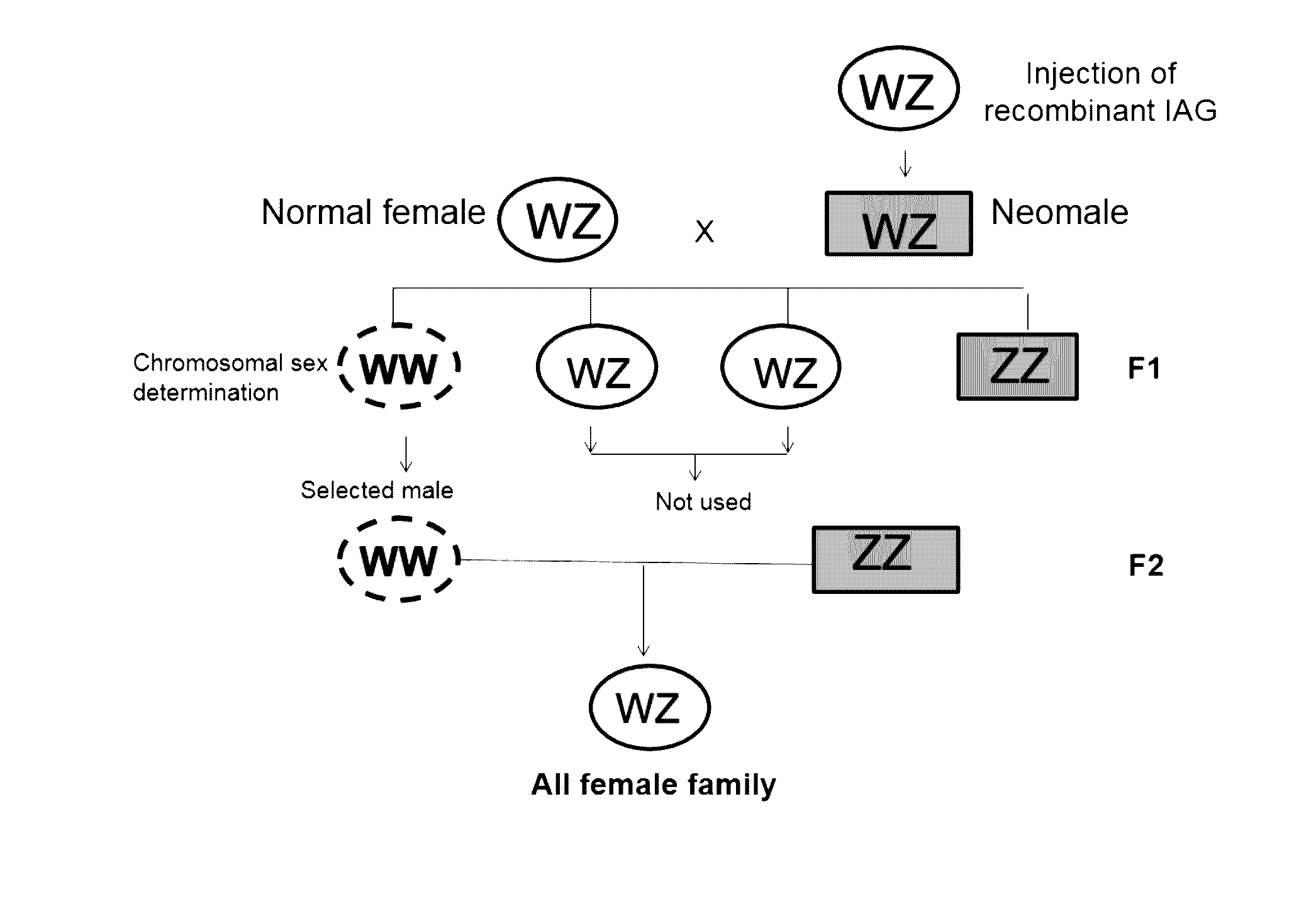
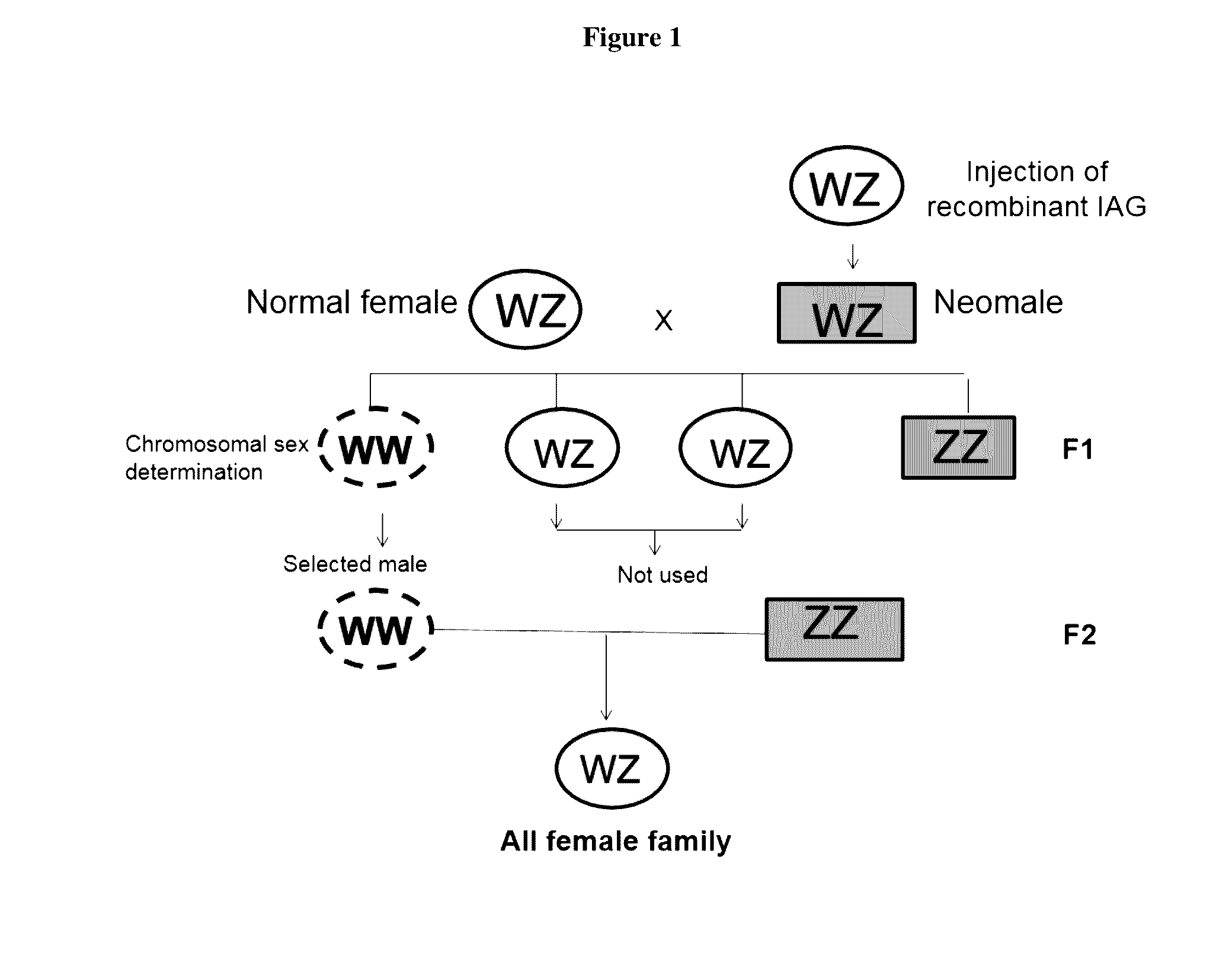
Moderation of crustacean androgenic gland peptide for mono sex culture
InactiveUS20150096499A1Induces silencingAlters sexual characteristicAnimal reproductionClimate change adaptationPresent methodChange sex
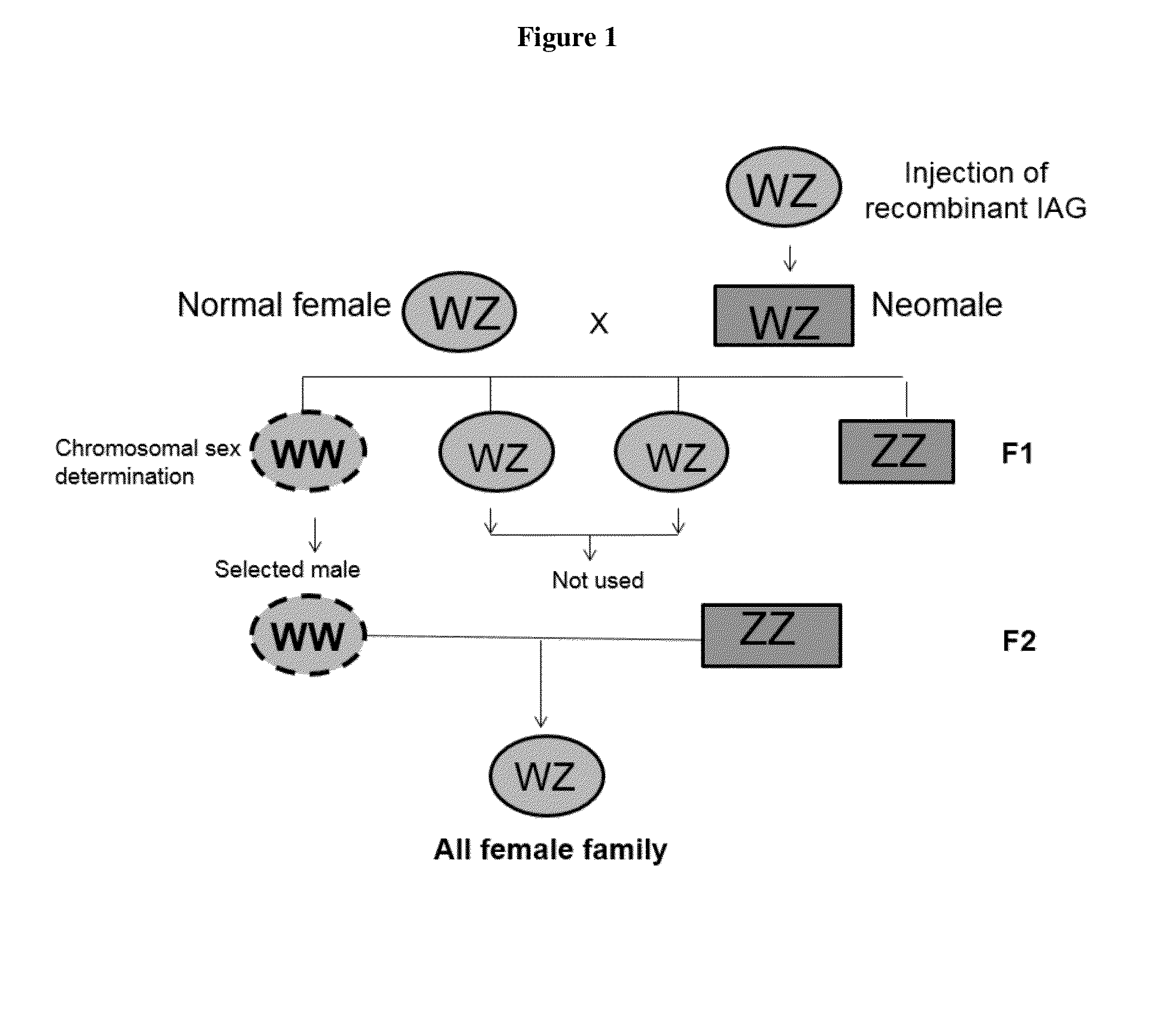
This invention is directed to methods for manipulating sex, reversing sex, changing sex ratio, or moderating crustacean androgenic gland peptide expression in crustaceans for producing a monosex culture. In one embodiment, the present method comprises the step of injecting a dsRNA for the gene of IAG into a male crustacean. In another embodiment, the present method comprises the step of injecting a dsRNA for the gene of IAG into a female crustacean that had acquired a spermatophore (a sperm sac) after mating with a male. In another embodiment, the present invention provides a method of producing a monosex culture of crustacean by introducing a recombinant IAG peptide into the female crustacean, thereby obtains male with altered sexual characteristics for mating with a normal female. In one embodiment, the crustacean is a shrimp or lobster.
Owner:CHAN SIU MING
Method for producing yy super-male and xy physiological female common carps
ActiveUS20180317459A1Easy and feasibleThe method is simple and feasibleAnimal reproductionOrganic active ingredientsNormal femaleCommon carp
Method for producing YY super-male and XY physiological female common carps, with which YY-chromosome super-male common carp can be cultivated and androgenetic YY super-male common carp is produced, where microsatellite markers are used for paternity testing and test crossing confirmation. The YY common carp is crossed with normal female common carp to produce the progenies of only male common carp, and without sex identification, the juvenile male common carp with known sex are subjected to artificial sex reversal to produce XY physiological female common carp.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Standard product for polyploid chromosome detection and preparation method thereof
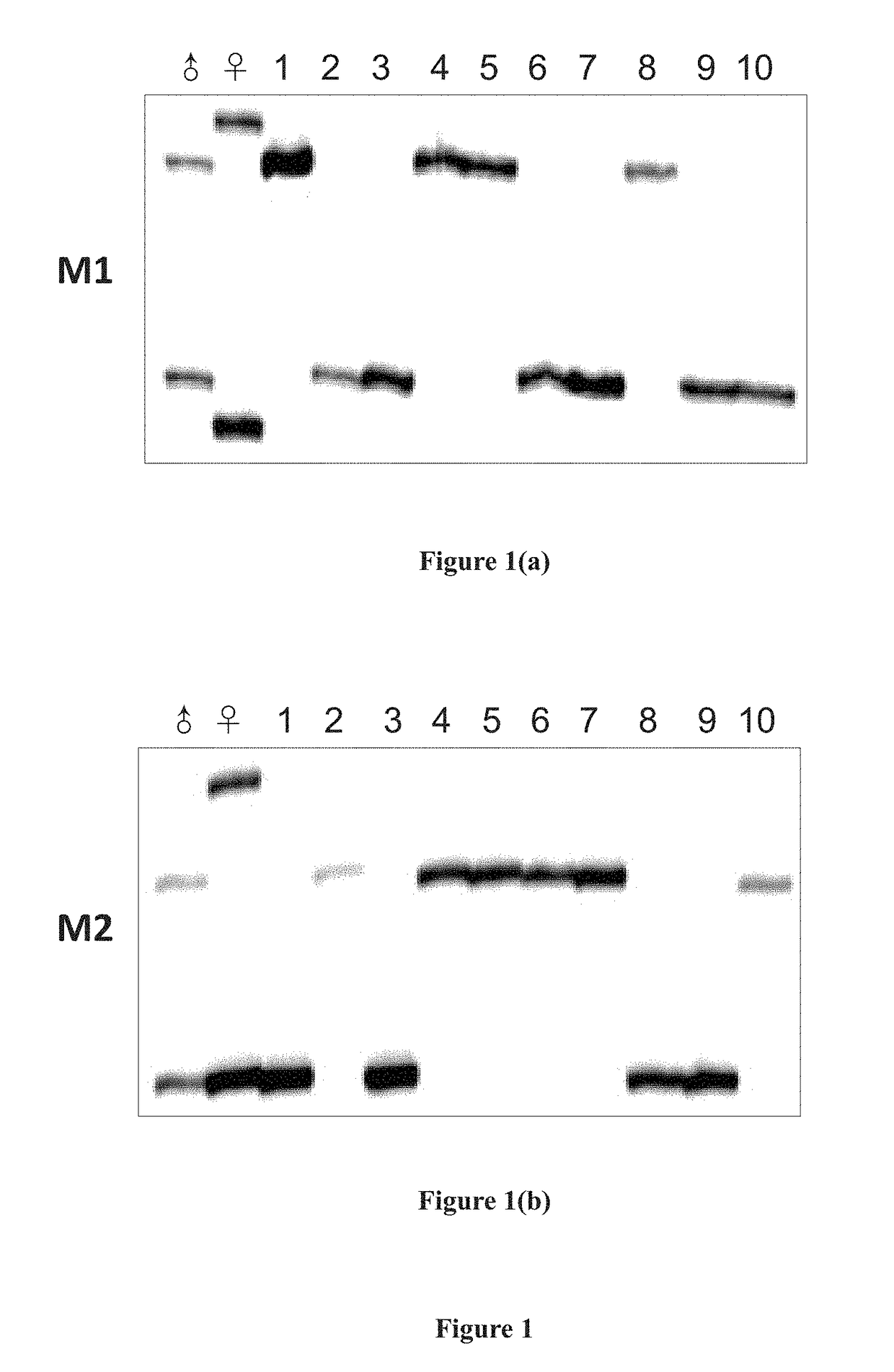
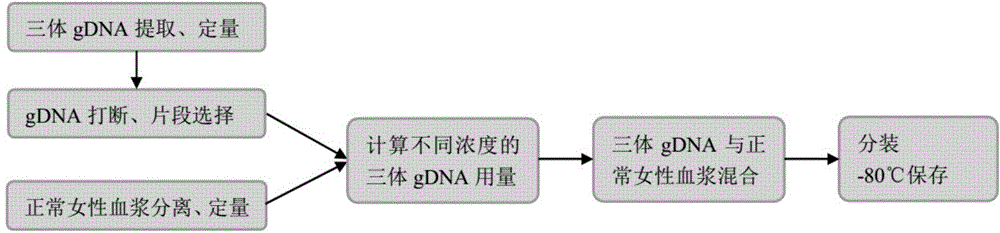
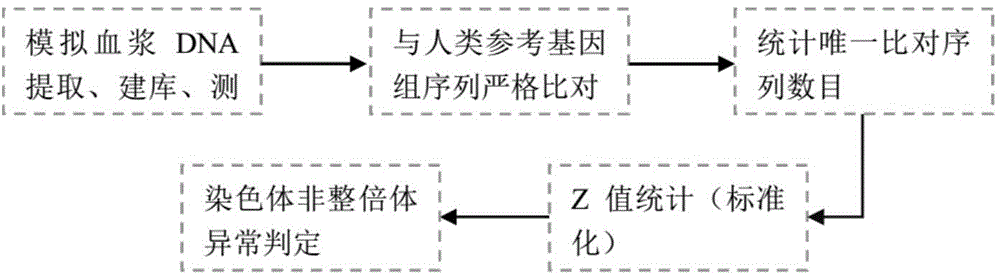
ActiveCN105821117ARich sourcesEasy to operateMicrobiological testing/measurementNormal femaleBlood plasma
The invention provides a standard product for polyploid chromosome detection and a preparation method thereof. The standard product comprises a first standard, a second standard and an optional third standard which are respectively positioned inside a separate container, wherein the first standard contains 3.5+ / -0.2% of polyploid chromosome gDNA fragments and residual normal female plasma on the basis of total mass (mass) of DNA in the standard; the second standard contains 4-6% of polyploid chromosome gDNA fragments and residual normal female plasma on the basis of total mass (mass) of DNA in the standard; and the first standard contains 8-12% of polyploid chromosome gDNA fragments and normal female plasma on the basis of total mass (mass) of DNA in the standard.
Owner:海南华大基因科技有限公司
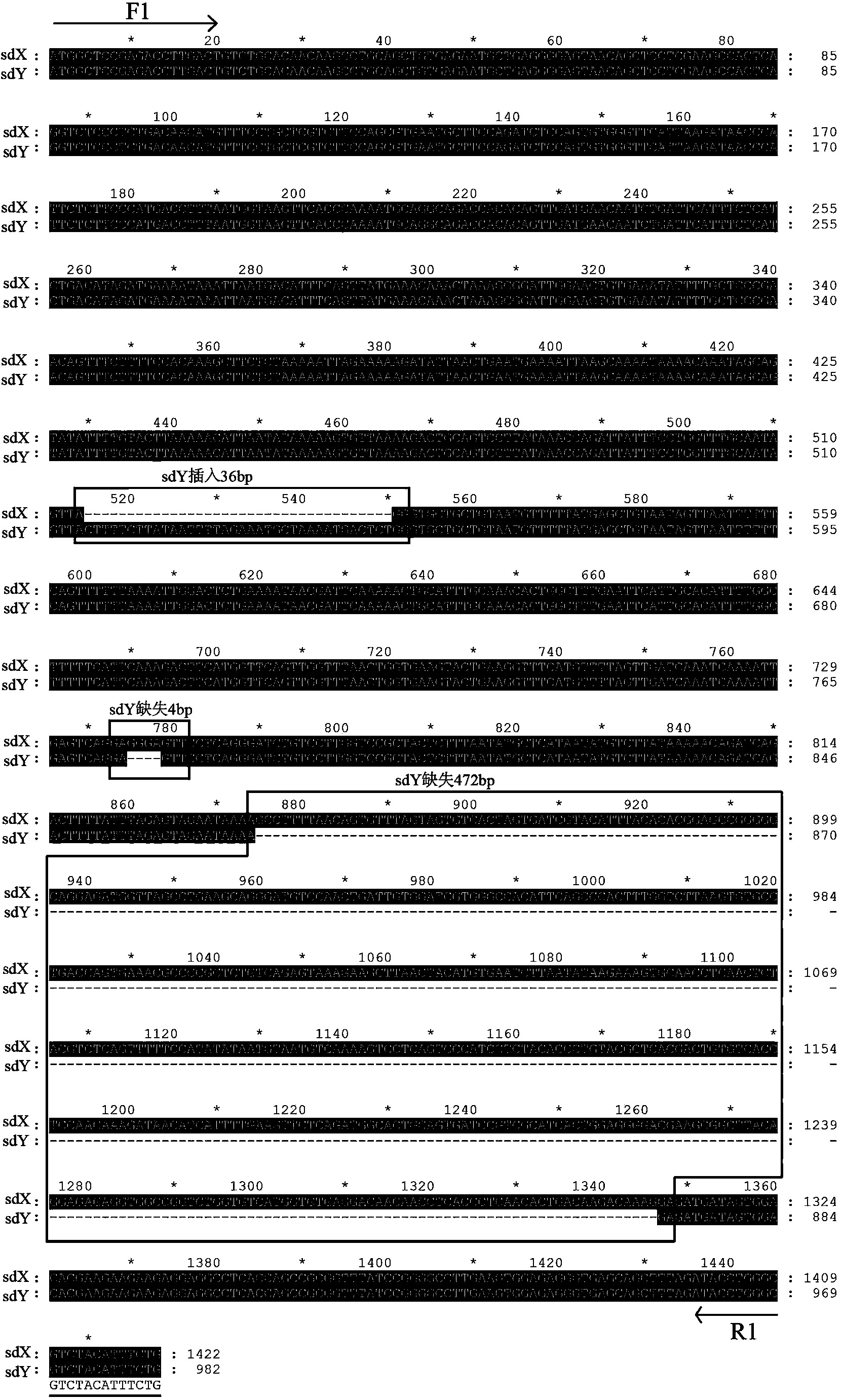
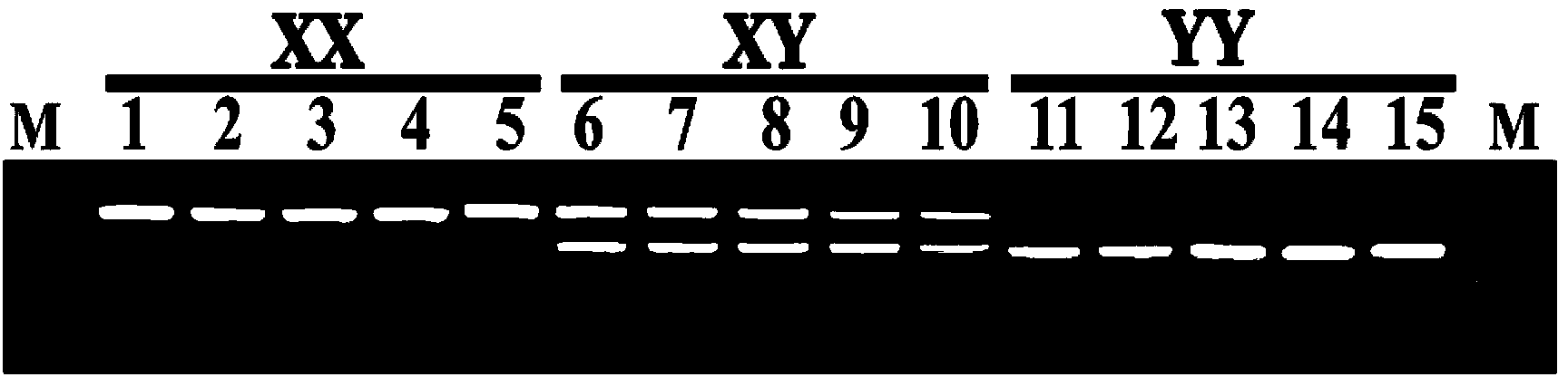
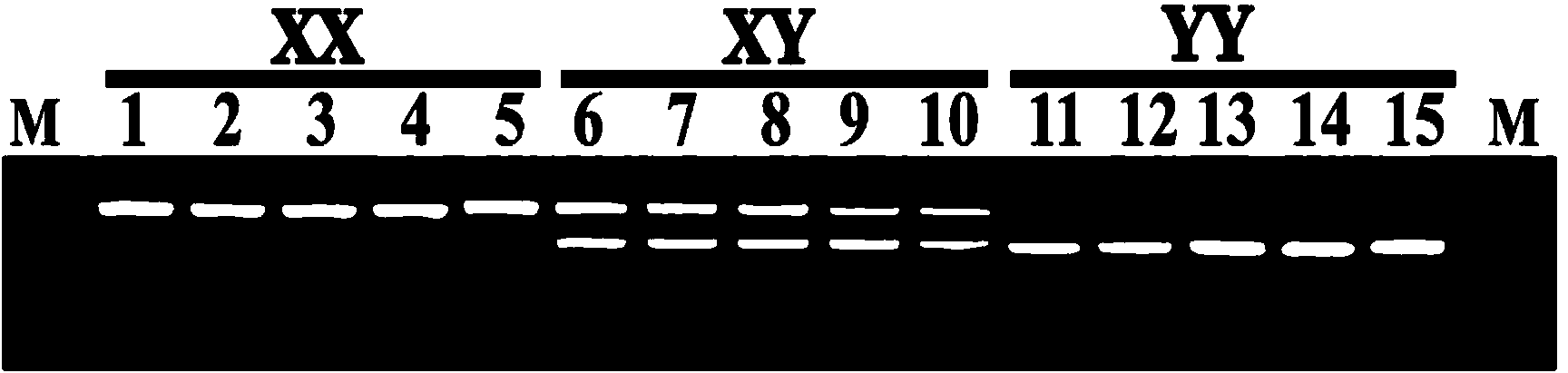
Y-chromosome specific molecular marker of Nile tilapia, and genetic sex determination method and supermale producing method both based on molecular marker
ActiveCN103409420AEconomic divisionQuick distinctionMicrobiological testing/measurementClimate change adaptationNucleotideNormal female
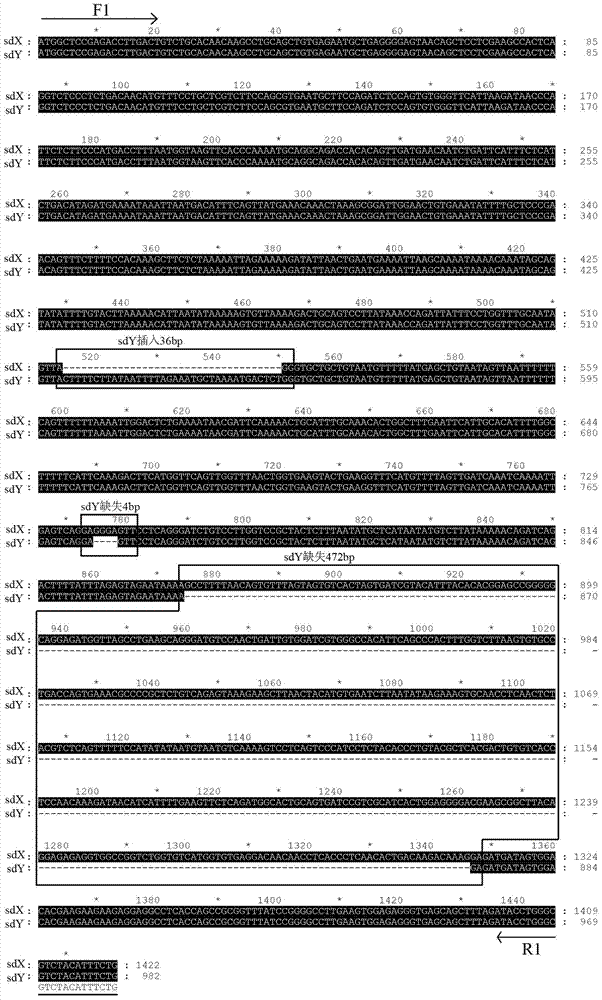
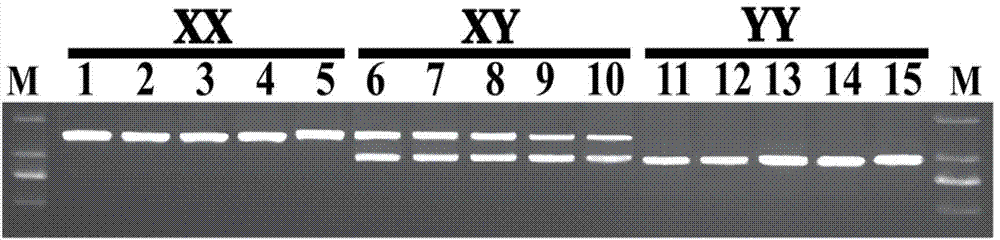
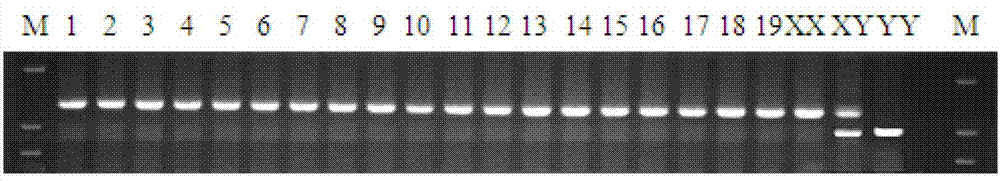
The invention discloses a Y-chromosome specific molecular marker of Nile tilapia, wherein the nucleotide sequence of the Y-chromosome specific molecular marker is as shown in SEQ ID No.4; the Y-chromosome specific molecular marker can be commonly used for a plurality of strains of Nile tilapia; a genetic sex determination method of Nile tilapia is established based on the molecular marker, so that normal female fish XX, normal male fish XY, converted female fish XY, converted female fish YY and supermale fish YY can be differentiated from each other economically, rapidly and accurately; large-scale production of supermale fish fry can be realized through mating of YY supermale fish and the normal female fish XX based on the genetic sex determination method; as a result, the yield of culture is improved remarkably, the culture cost is reduced and the economic benefit is enhanced; besides, a method of producing the YY supermale fish through mating of the supermale fish YY and the converted female fish YY is also established by using the Y-chromosome specific molecular marker.
Owner:SOUTHWEST UNIV
Cultivation method of polyploid pomegranate
InactiveCN103392606AReduce incubation timeOvercome time-consuming disadvantagesHorticulture methodsPlant tissue cultureHybrid seedNormal female
The invention relates to a cultivation method of polyploid pomegranate. The cultivation method comprises following steps: selecting branched flower buds and branches which grow on the top of a diploid plant, inducing the flower to generate polyploid pollen by dropwise adding chemical materials into the flower buds, carrying out hybridizations between induced male flowers and normal female flowers or between induced female flowers and normal male flowers so as to obtain polyploid hybrid fruits, culturing the hybrid seeds in the hybrid fruits so as to obtain polyploid plants, and culturing the polyploid plants in a tissue cultivation mode so as to obtain polyploid pomegranate plants. The cultivation method overcomes the shortage that a large amount of time is consumed by the conventional chromosome reduplication methods. The cultivation method utilizes the fact that the pollen and ovule cells in the reproductive organs are haploids, namely 1n chromosome, during the pomegranate blossom period, uses chemical materials to process the pollen and ovule cells to induce chromosome doubling, thus largely reduces the time of chromosome doubling process, and has the advantages of available materials, simple operation, and high success rate.
Owner:南京大渊美容保健有限公司
Moderation of crustacean androgenic gland peptide for mono sex culture
InactiveUS20170035034A1Induces silencingAlters sexual characteristicAnimal reproductionClimate change adaptationPresent methodChange sex
Owner:CHAN SIU MING
Embryo transferring model and teaching method thereof
ActiveCN108597337AEasy to controlIntuitive and Accurate UnderstandingEducational modelsUterine bodyExternal catheter
The invention discloses an embryo transferring model and a teaching method thereof. According to the proportion of a normal female reproductive system, the lengths, widths, thicknesses, volumes and diameters of all parts (the uterine body cavity, cervix and vagina) of a model are specifically quantitatively designed, combined with the lengths of transferring internal and external catheters, an embryo experimentalist and a clinicist can more intuitively and more accurately understand the relative distance and space of a transferring path, thus the embryo transferring position is better controlled, the clinicist can fully feel certain key difficulties in the transferring path, through repeated practice, the difficulties are overcome in formal transferring operation, and smooth intubation isachieved.
Owner:ZHEJIANG UNIV
Method for continuously producing complete male pelteobagrus fulvidraco by sex reversion and embryonic development
InactiveCN1994072BShorten breeding timeImprove ergonomicsAnimal reproductionClimate change adaptationNormal femaleFishery
The invention relates to a method for producing male fish, wherein it comprises that: artificially inducing; artificially inducing the female and male fishes; artificial insemination; inverting hormone; feeding seed with Artemia which contains hormone; converting male into biological female; the female grows. The invention comprises sperm inactivation and female diploid generation, to generate ready male fish and normal female fish; crosses the male fish and female fish to obtain the full male fish; inverts the male fish into biological female fish to cross with male fish, to obtain the full super male fish.
Owner:INST OF AQUATIC LIFE ACAD SINICA
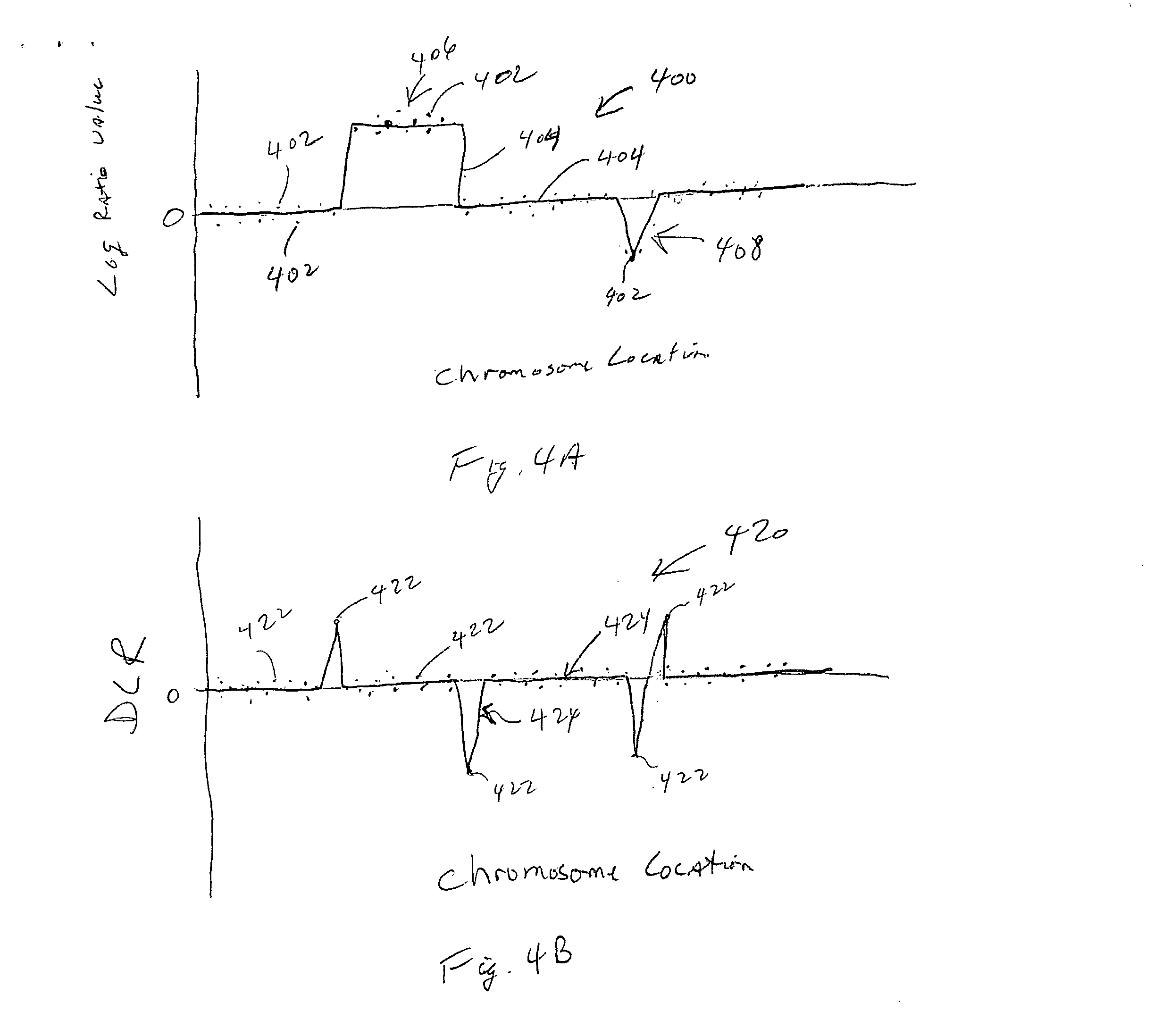
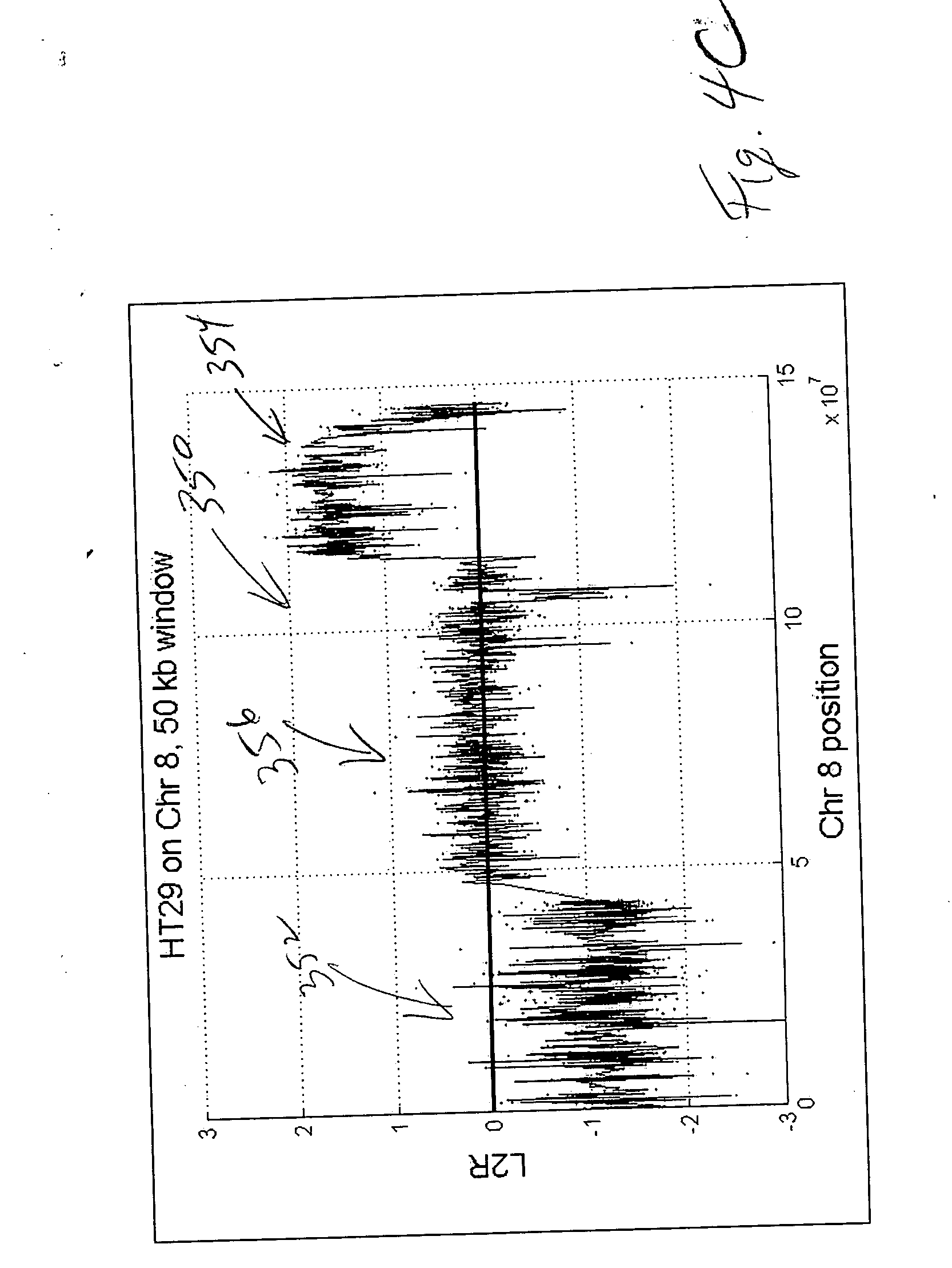
CGH array quality assessment
ActiveUS20070082338A1Quality be determineMicrobiological testing/measurementBiological testingNormal femaleReceiver operating characteristic
Methods, systems and computer readable media for characterizing quality of CGH arrays, including generating at least one metric for a CGH array that characterizes a property of the CGH array; repeating generation of the at least one metric for at least one additional CGH array; and comparing each metric across all CGH array metric values generated to identify a quality of any one of the CGH arrays characterized, relative to the CGH arrays characterized for that metric. Methods, systems and computer readable media for determining the quality of a CGH array, including calculating a spread of the derivative of log ratio value differences between consecutive probes representing consecutive positions along a chromosome, wherein ratio values are calculated from probe signals from a CGH array, as read by a reader on first and second channels thereof, or from probes signals from two CGH arrays, as read by a single channel reader. Methods, systems and computer readable media for qualifying a CGH array, including quantifying an interarray reproducibility metric characterizing selected sets of probe replicates. Methods, systems and computer readable media for qualifying a CGH array, including generating a Receiver Operating Characteristic (ROC) curve and analyzing false positive and true positive signals from a normal male sample and a normal female sample, as read by a reader on first and second channels thereof, or from one of a normal male sample and a normal female sample on the CGH array and the other of the normal male sample and normal female sample on a second CGH array, as read by a single-channel reader. Methods, systems and computer readable media for determining the quality of a CGH array, including segmenting, by copy number, log ratio signals from probes on the CGH array representing a genome; plotting a probability distribution of the segmented log ratio values; and quantifying separation between copy number peaks resulting from the plotting of the probability distribution.
Owner:AGILENT TECH INC
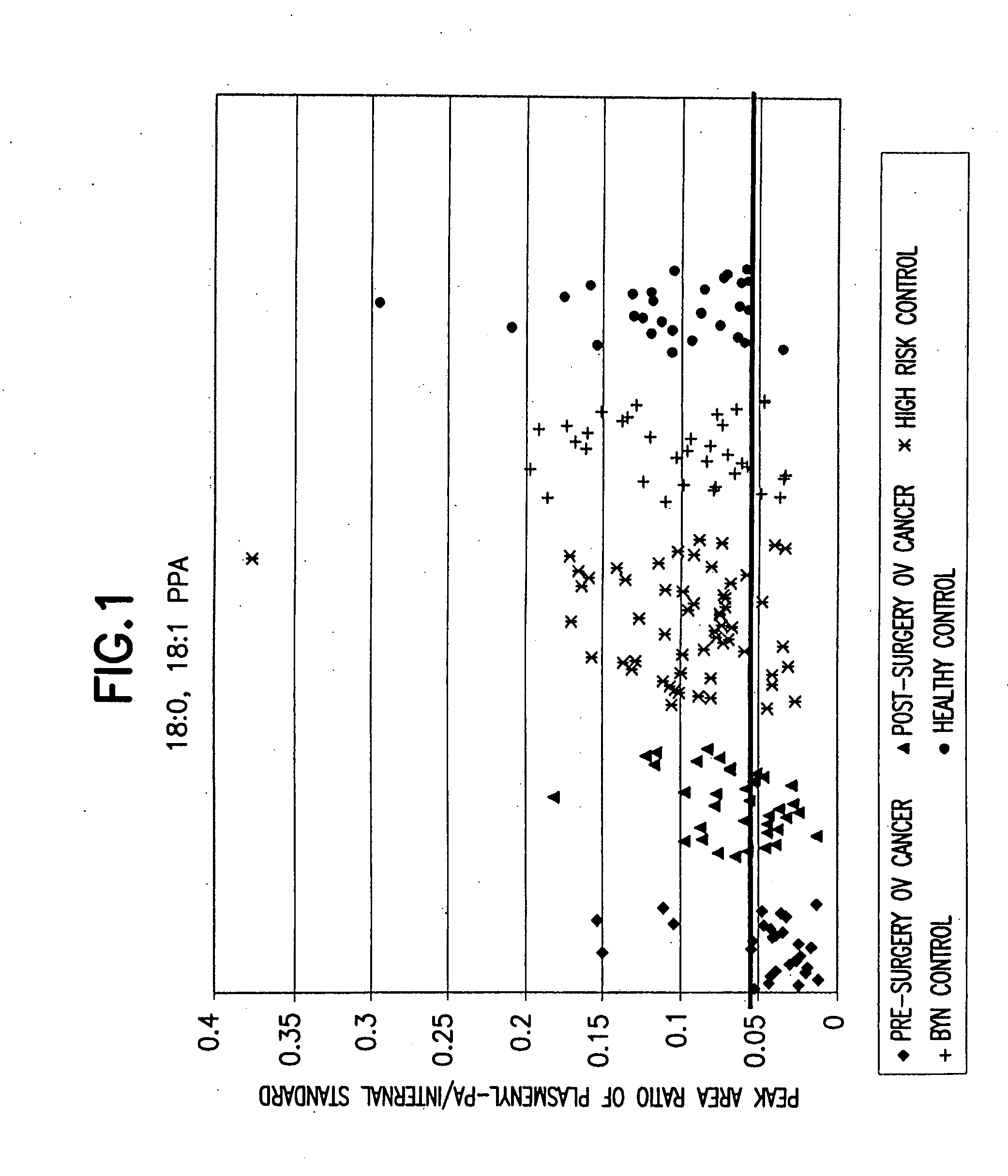
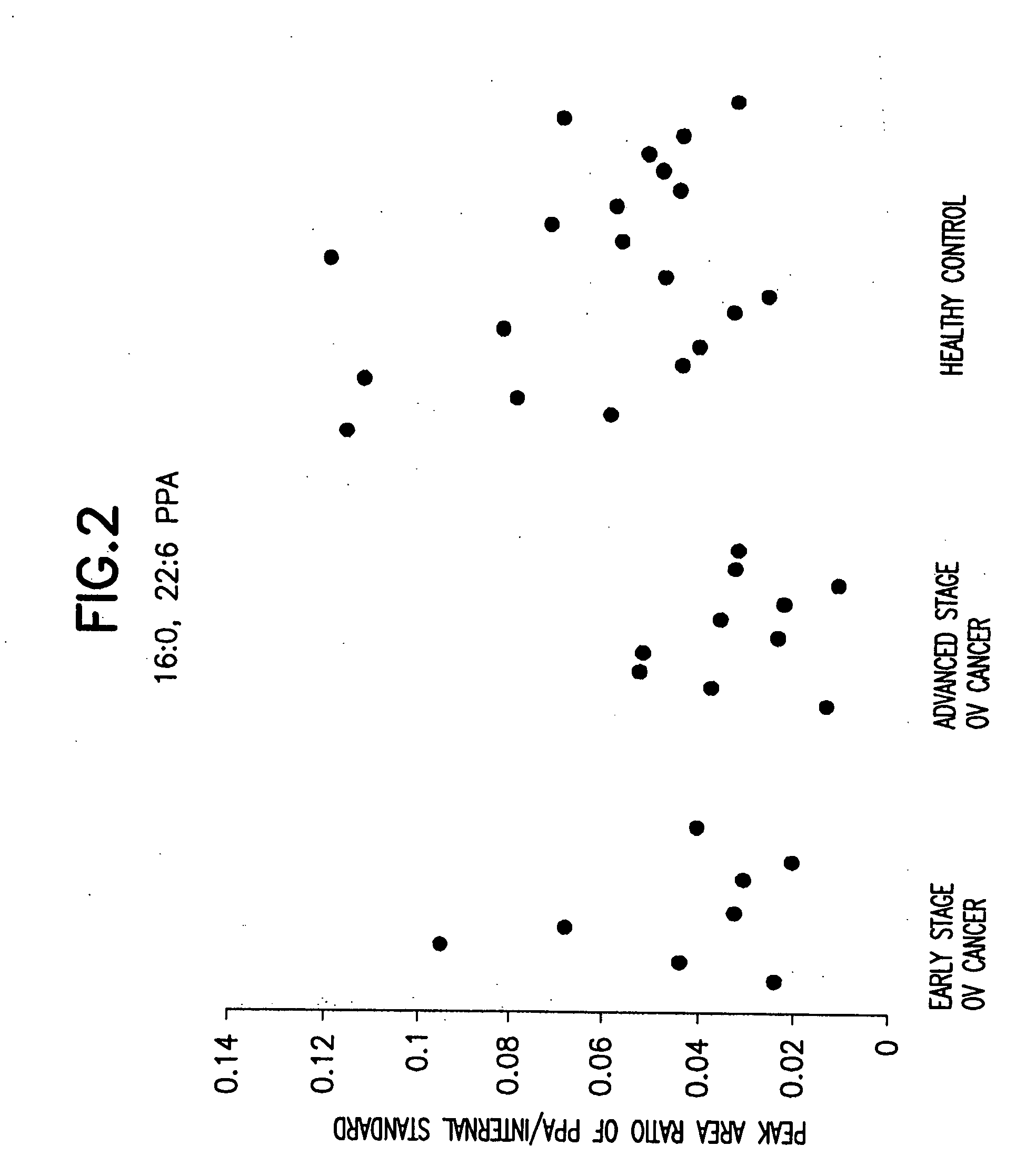
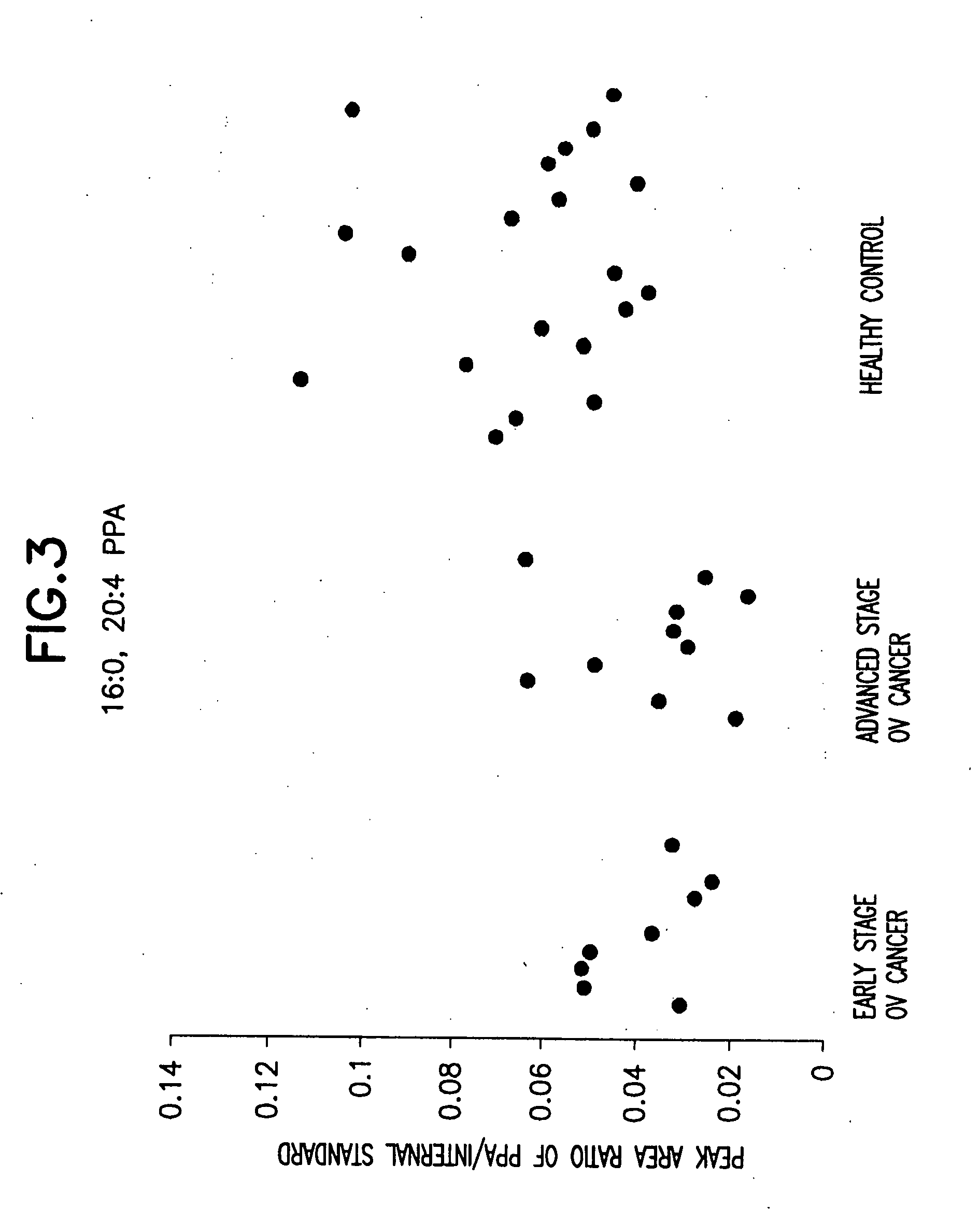
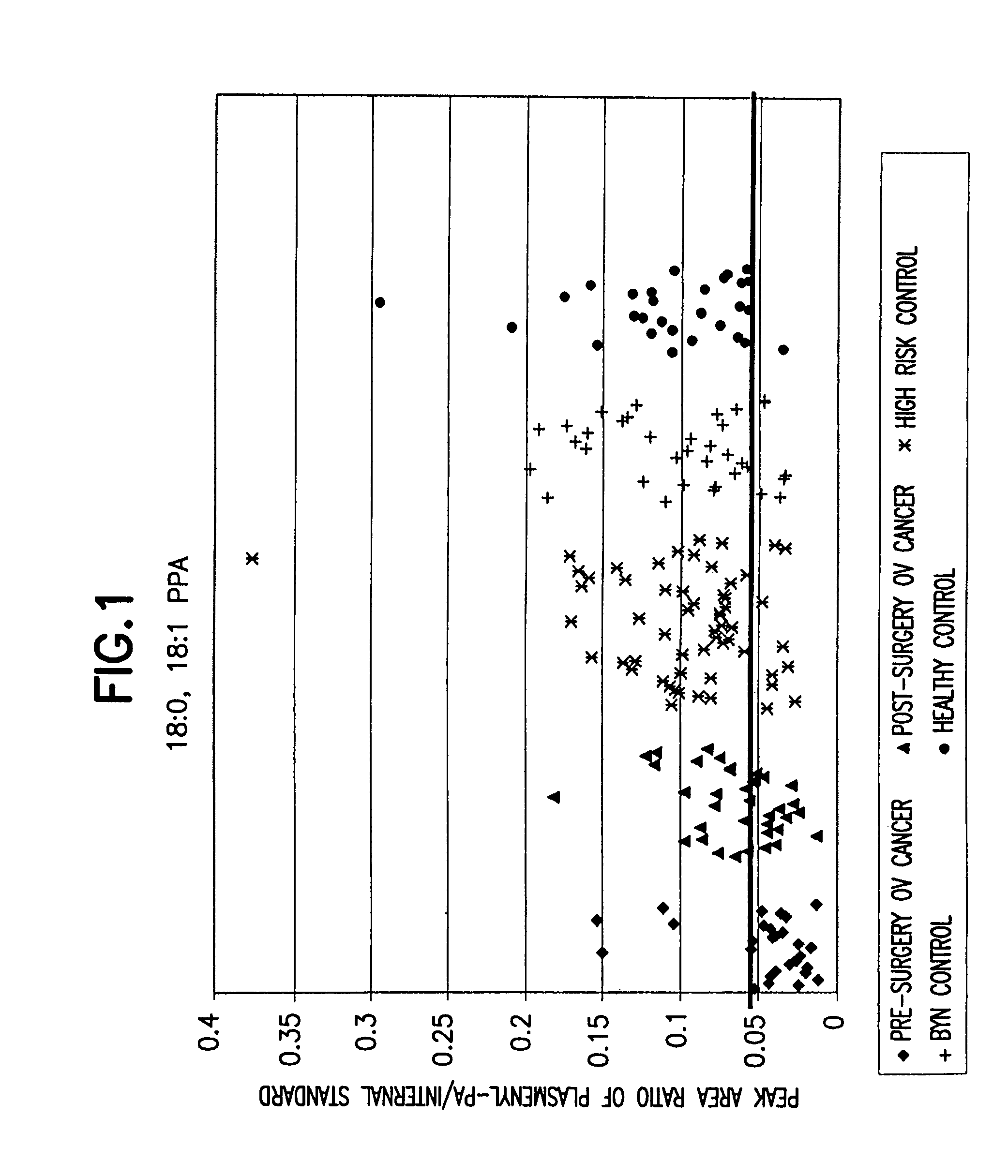
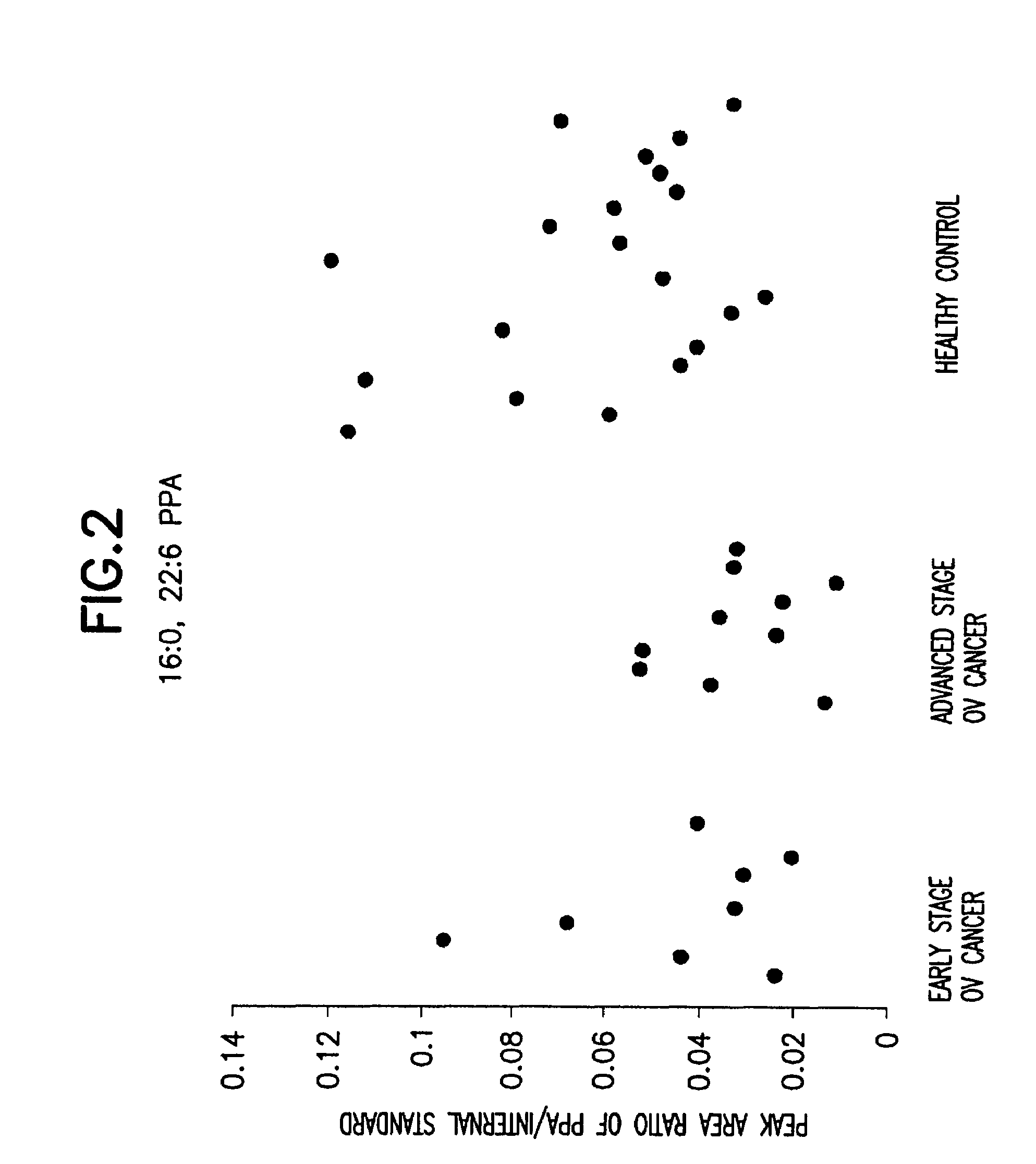
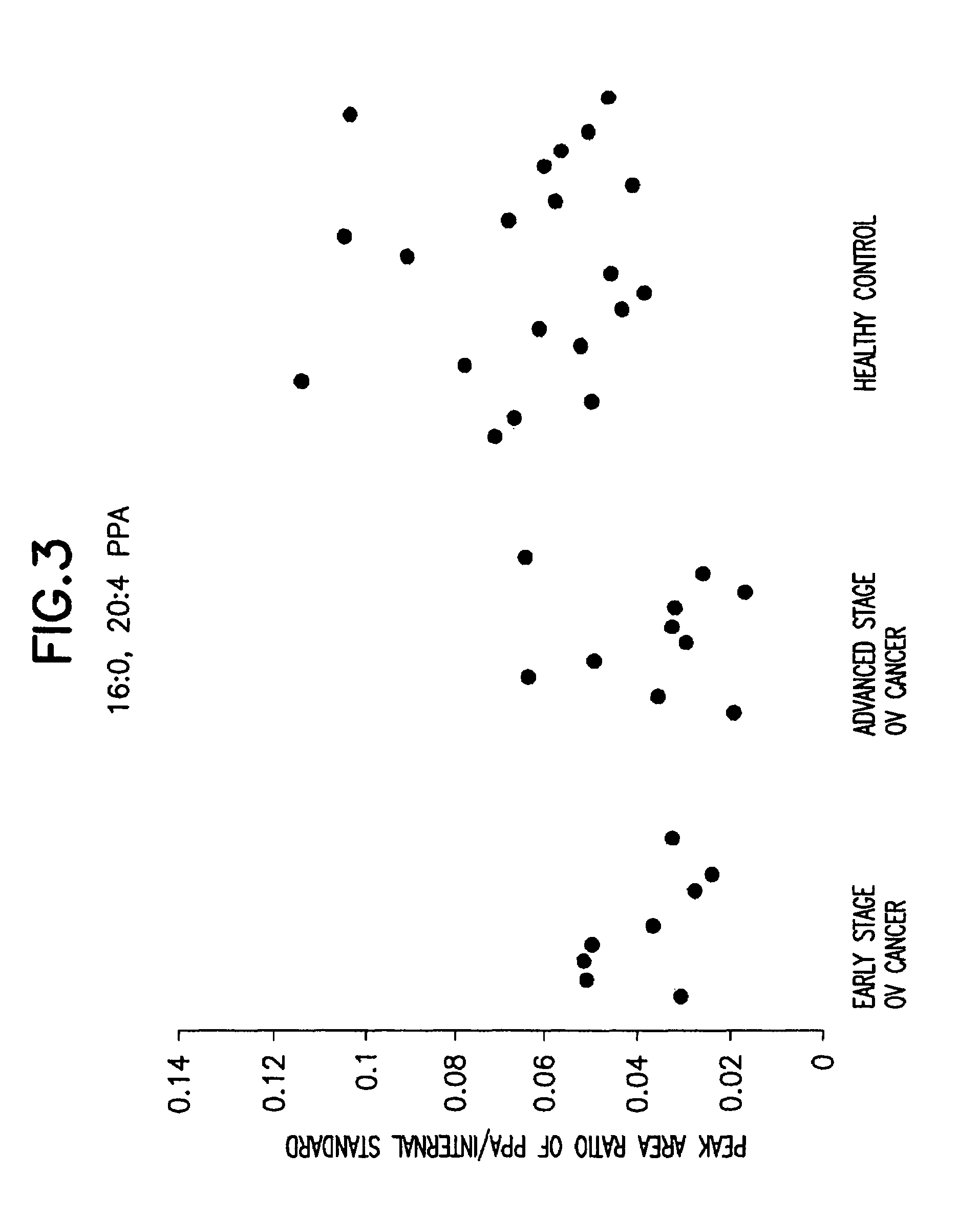
Method for detecting ovarian cancer
A method of detecting ovarian cancer in a female test subject comprising determining the amount of plasmenyl-PA or a biomarker having a mass charge ratio of approximately 655.3 in a sample of a bodily fluid taken from the female test subject and comparing the amount of plasmenyl-PA (or the biomarker) in the sample of the bodily fluid taken from the female test subject to a range of amounts of plasmenyl-PA (or the biomarker) found in samples of bodily fluids taken from a group of normal female subjects of the same species as the female test subject and lacking ovarian cancer, whereby a lower amount of the plasmenyl-PA (or the biomarker) in the sample of the bodily fluid taken from the female test subject indicates the presence of ovarian cancer.
Owner:THE CLEVELAND CLINIC FOUND +1
X-chromosome specific molecular marker of Nile tilapia
ActiveCN103409421ARealize large-scale productionIncrease productionMicrobiological testing/measurementDNA/RNA fragmentationX chromosomeNormal female
The invention discloses an X-chromosome specific molecular marker of Nile tilapia. The nucleotide sequence of the X-chromosome specific molecular marker is as shown in SEQ ID No.3; the X-chromosome specific molecular marker can be commonly used for a plurality of strains of Nile tilapia; the genetic sex determination of the Nile tilapia can be performed economically, rapidly and accurately based on the molecular marker; the molecular marker is advantageous for establishing a method for producing YY supermale fish through mating of supermale fish YY with converted female fish YY; therefore, large-scale production of all male fish fry can be realized through mating of the YY supermale fish and the normal female fish XX; as a result, the yield of culture is improved remarkably, the culture cost is reduced and the economic benefit is enhanced.
Owner:SOUTHWEST UNIVERSITY
Papaya plants having a mutant allele for hermaphroditism
ActiveUS20140237678A1Mutant preparationOther foreign material introduction processesPapaya familyMapinguari
Papaya plants containing mutant allele EWSMHP, which confers production of highly hermaphroditic progenies upon selfing of its hermaphrodite plants and also production of highly hermaphroditic F1 progenies when crossed with normal female and normal hermaphrodite papaya plants, are disclosed. The invention relates to the seeds of papaya plants having mutant allele EWSMHP, to the plants and plant parts of papaya plants having mutant allele EWSMHP and to methods for producing progeny of papaya plants having mutant allele EWSMHP. The invention also relates to methods for producing a papaya plant having mutant allele EWSMHP containing in its genetic material one or more transgenes and to the transgenic papaya plants and plant parts produced by those methods. The invention also relates to papaya cultivars or breeding cultivars, and plant parts derived from papaya plants having mutant allele EWSMHP. The invention further relates to hybrid papaya seeds, plants, and plant parts produced by crossing a plant having mutant allele EWSMHP with another papaya cultivar.
Owner:HORTIGENETICS RES S E ASIA
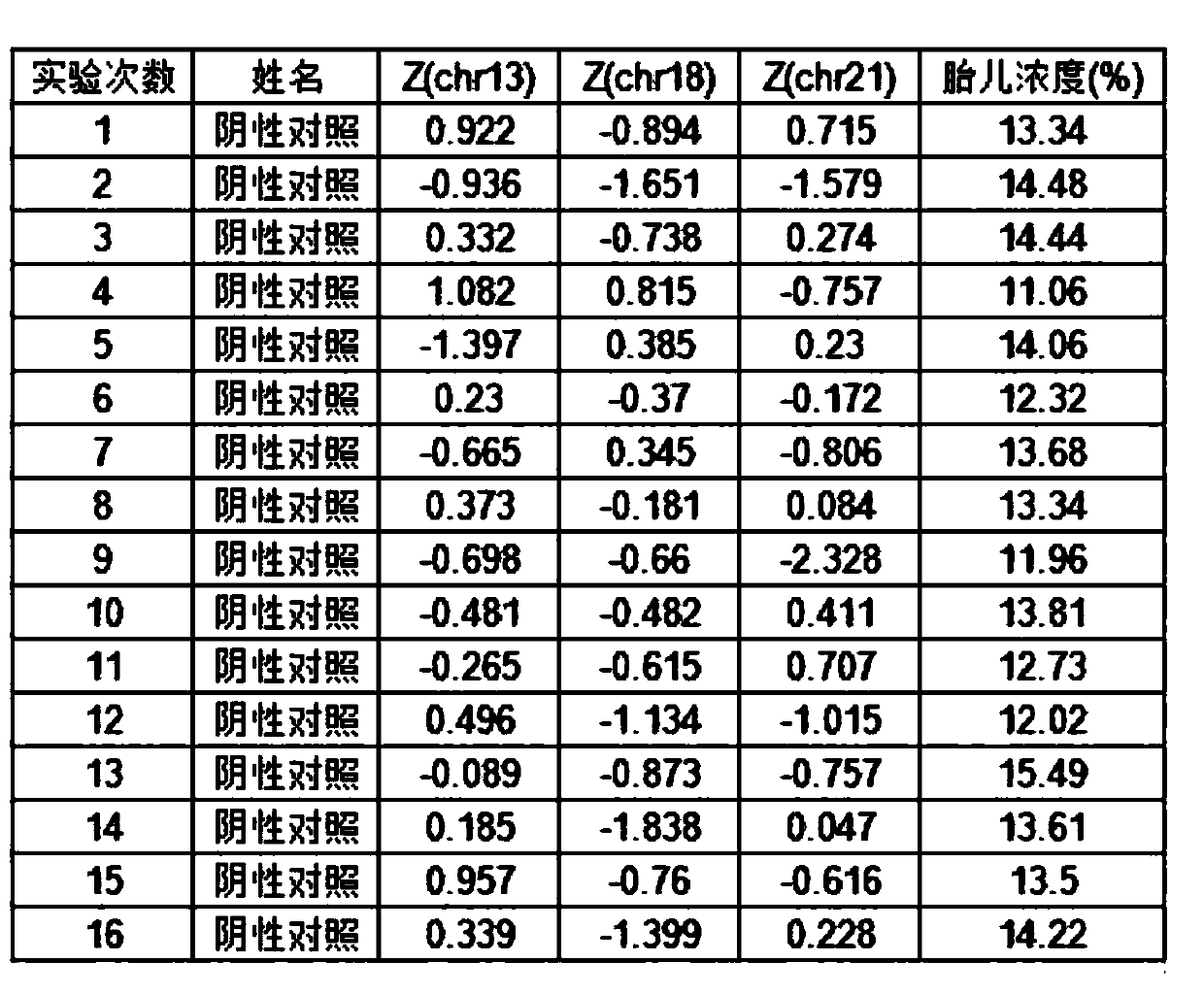
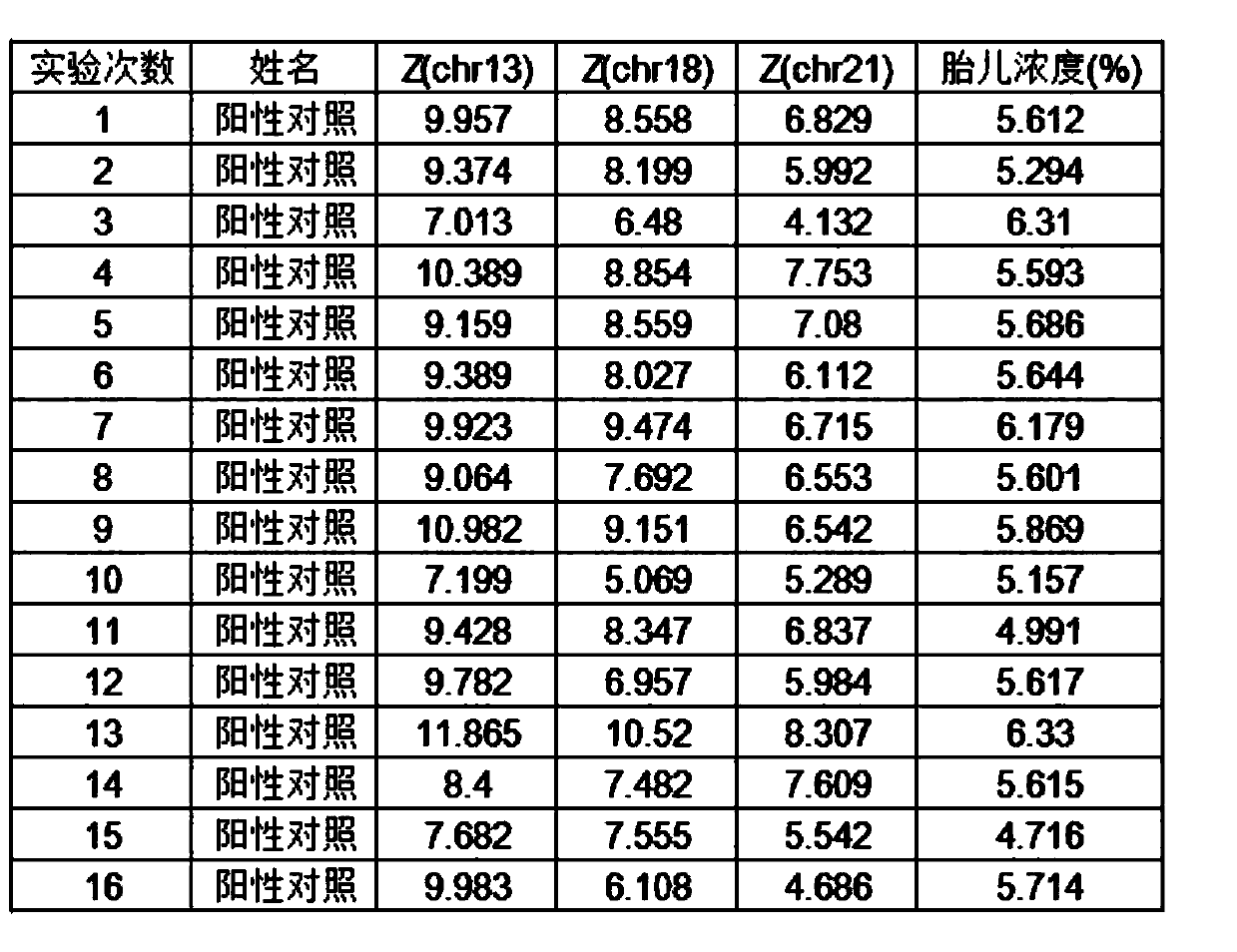
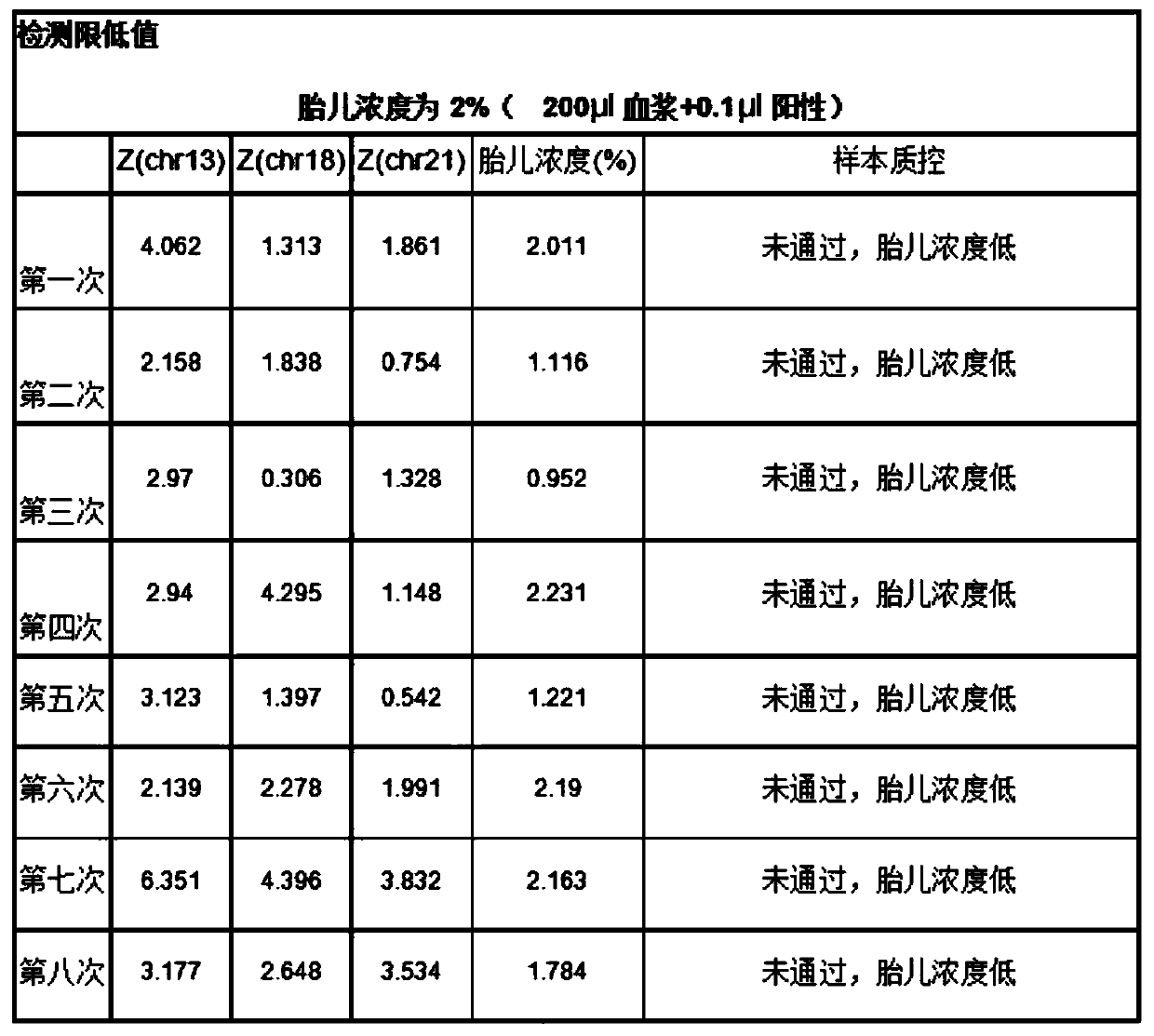
Quality control plasma for noninvasive prenatal detection and preparation method thereof
ActiveCN111440860AAlleviate the downside of difficult accessMicrobiological testing/measurementGynecologyNormal female
The invention relates to quality control plasma for noninvasive prenatal detection and a preparation method thereof. The quality control plasma comprises positive quality control plasma, negative quality control plasma and detection limit-type quality control plasma; the positive quality control plasma comprises a mixed solution which is composed of a positive quality control product of a verifiedkit and stored pregnant woman plasma with a low-risk non-invasive prenatal detection result and a normal female fetus after birth; the negative quality control plasma comprises a stored pregnant woman plasma solution with a negative non-invasive prenatal detection result and a normal female fetus after birth; and the detection limit-type quality control plasma comprises a mixed solution of plasmaof a healthy non-pregnant woman and the positive quality control product of the verified kit. The method has the advantages that: a mixed solution of negative specimen plasma of the pregnant woman and the positive quality control product of the verified kit is used for replacing existing positive specimen plasma, and due to the fact that negative specimen plasma is more, the defect that the positive specimen plasma is difficult to obtain can be greatly relieved.
Owner:SHAOXING WOMEN & CHILDRENS HOSPITAL +1
Method for cultivating ZW type lepidopteraus pest linkage balanced lethal system using molecule genetic label
InactiveCN1561712AGet rid of restrictionsAvoid ineffective laborMicrobiological testing/measurementAnimal husbandryZ chromosomeFluorescence
A method for using molecular genetic marker to culture ZW-type sex-linked balance lethal line of lepidopterous pests includes searching the molecular marker on chromosome Z of target pest, using it as translocation screening marker, gamma ray radiating to induce W-Z chromosomes' translocation, screening the translocated female individual, gamma ray radiating to male anjd female pests for inducing recessive lethal mutation or intercross to generate generation F1, using its male pest to mate with normal female pest and then with translocated female pest, detecting if there is living female pest in generation F2, synchronously screening two excessive lethanl genes, and culturing sex-linked balance lethal line.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Human placental site trophoblastic tumor cell line
The invention belongs to the field of microbial animal cell lines, and in particular relates to a human placental site trophoblastic tumor cell line and a constructing method of the human placental site trophoblastic tumor cell line. Through the in-vitro primary culture on the focus in the uterus of patients suffering from placental site trophoblastic tumor, the human placental site trophoblastic tumor cell line is established; the human placental site trophoblastic tumor cell line is named PSTT-1, and is assigned with the accession number of NO.10882. The human placental site trophoblastic tumor cell line has the biological characteristics that the cells grow in single layer in a wall-attaching manner, the contact inhibition is eliminated, the state is nonuniform, and the cells are in the star-shaped or fusiform-shaped flat structure with multiple bulges; the growth in in-vitro culture is good, and the stable and continuous passage for more than 30 generations can be realized; the karyotype analysis result is the normal female karyotype: 46, XX. The PSTT-1 cell line can be used for the research on the nosetiology, the transfer mechanism, the drug-resistant mechanism and the like of the placental site trophoblastic tumor diseases.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV +1
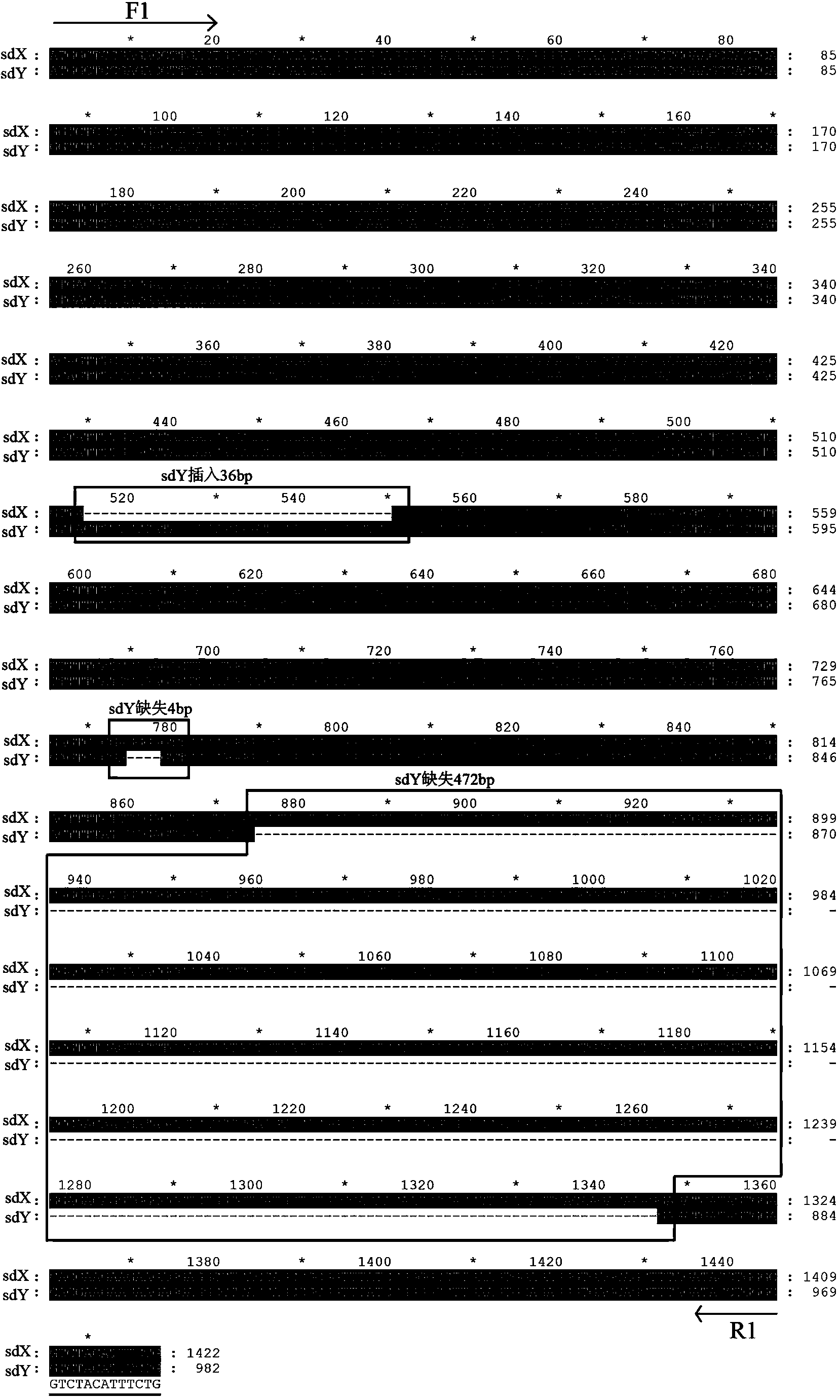
Specific molecular markers of sex chromosomes of silurus meridionalis and genetic sex identification method and unisexual fish production method based on molecular markers
ActiveCN110551808AEconomic divisionQuick distinctionMicrobiological testing/measurementClimate change adaptationSilurusSilurus meridionalis
The invention relates to specific molecular markers of sexes of silurus meridionalis and a silurus meridionalis genetic sex identification method and a unisexual fish production method based on the molecular markers. The invention screens specific molecular markers of X and Y chromosomes of silurus meridionalis for the first time. A silurus meridionalis genetic sex PCR identification method established based on the molecular markers has the characteristics of high efficiency, accuracy and stability, can be applied to wild silurus meridionalis in laboratories and different watersheds, and can economically, quickly and accurately distinguish normal female fishes, normal male fishes, transformed female fishes, transformed male fishes and super-male fishes, thereby realizing large-scale production of complete female fish fries, significantly increasing the breeding yield, reducing the breeding cost, and improving economic benefits. Furthermore, a large number of unisexual fish fries can beobtained so as to be applied to fundamental research.
Owner:SOUTHWEST UNIVERSITY
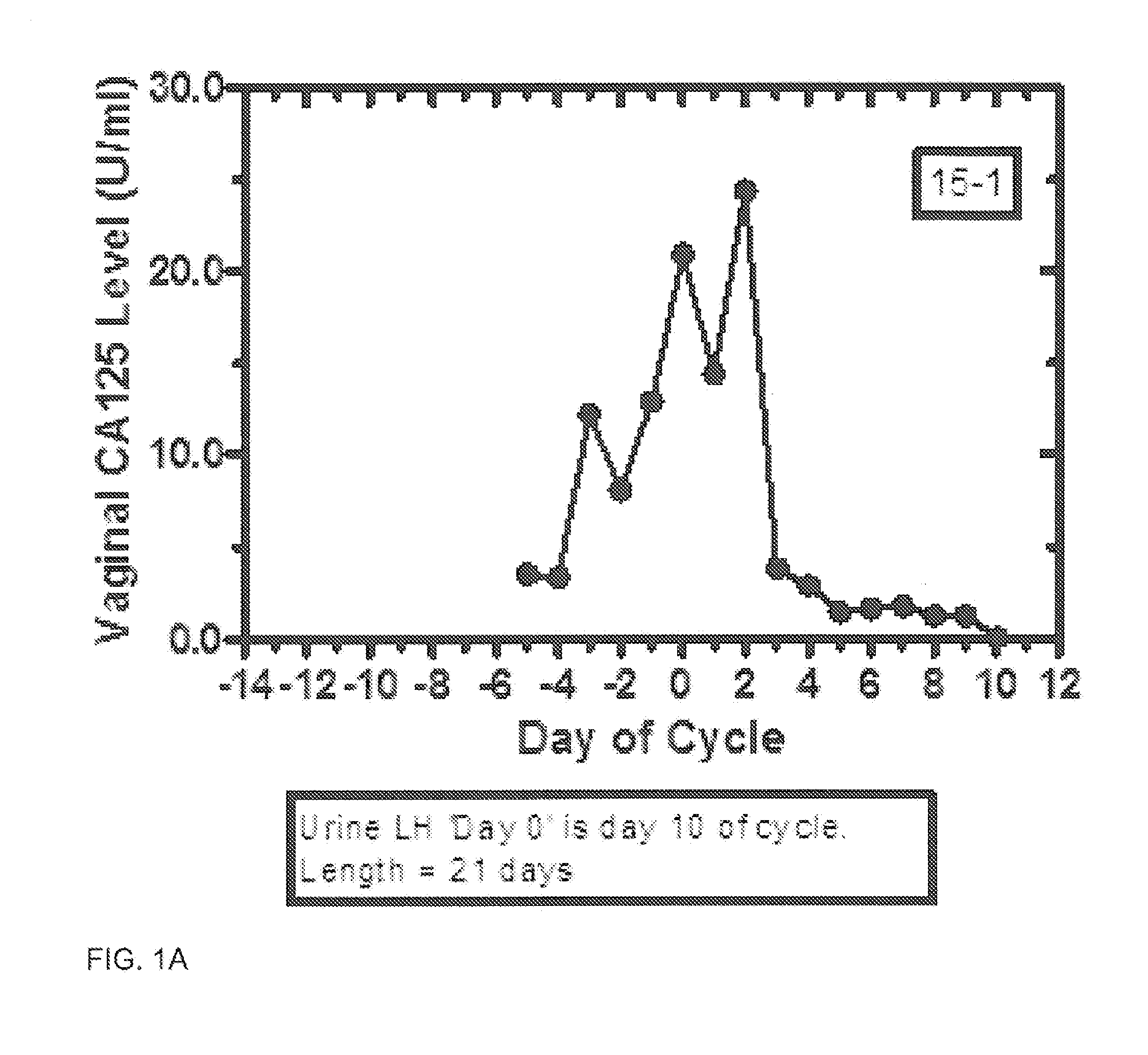
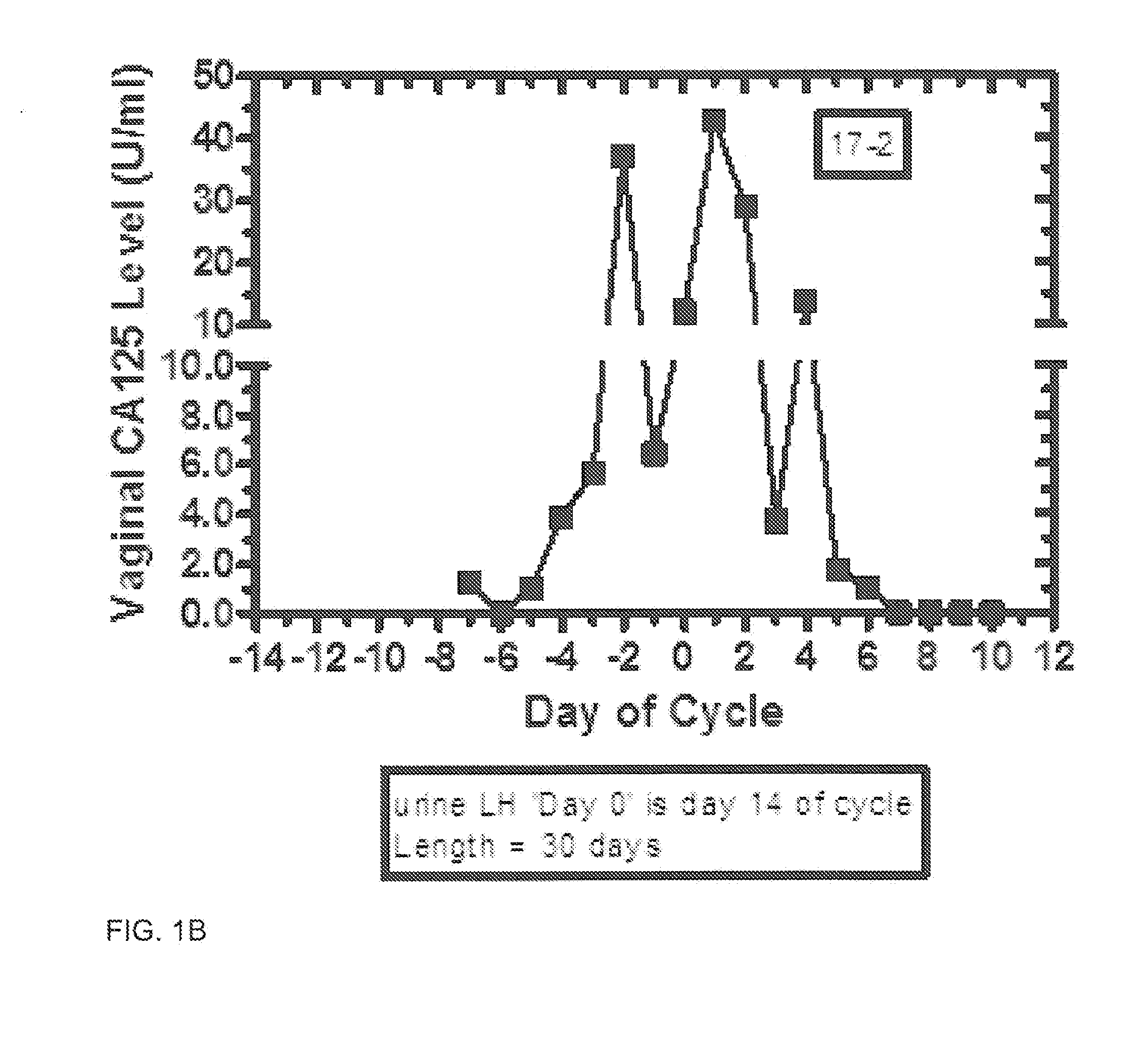
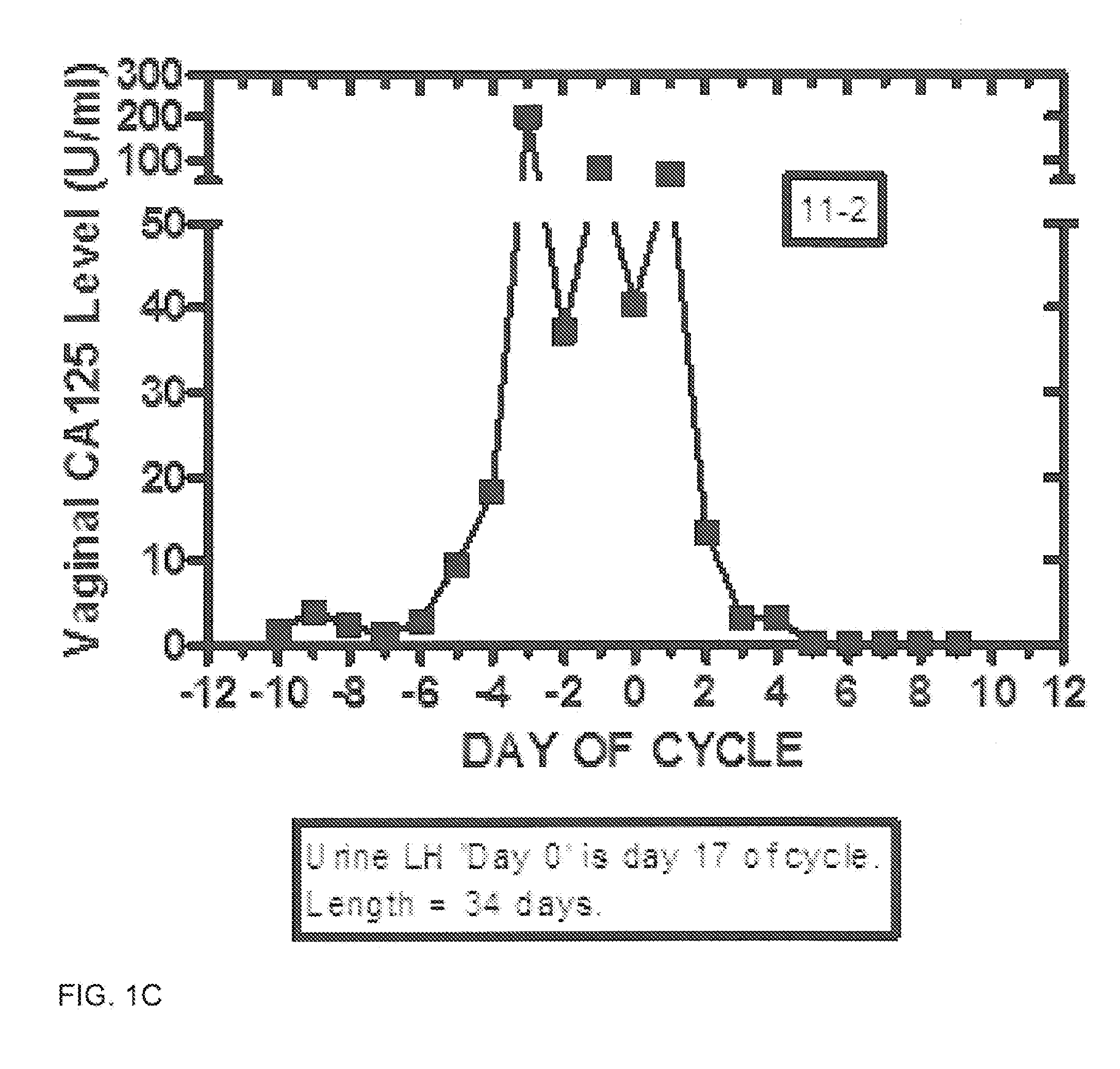
Methods and kit system for assessing fertility with vaginal CA125/MUC16 levels
Disclosed are methods for assessing the period of potential fertility, days of maximum fertility, and transition to the infertile phase via measurement and processing of vaginal CA125 (MUC16) levels. In one embodiment, the fertile and infertile phases of the ovulatory normal female menstrual cycle are defined by a pattern or signature of day-specific vaginal CA125 levels. Suitably, signatures are based upon daily cotton swab collection of vaginal epithelial and fluid plus subsequent assay of CA125. Day-specific vaginal CA125 assay levels increase during the period of potential fertility and reach a maximum with inflection point generally before and near the time of ovulation. Derived functions of day-specific vaginal CA125 are used to predict fertility, enhance conception, and for birth control.
Owner:USALA STEPHEN J +2
Orthotic device for female legs and knees
InactiveCN103815998ACurvaceous toCurve beauty hasElectrotherapyMagnetotherapyNormal femaleEngineering
The invention relates to an orthotic device for female legs and knees. The orthotic device is characterized by comprising a body, wherein the body is fixedly connected with a magnet sheet; the upper end of the magnet sheet is fixedly connected with a magnetic interlayer, and the lower end of the magnet sheet is fixedly connected with a demagnetization layer; a long waist-shaped hole is formed in the middle part of the demagnetization layer; the two sides of the body are provided with telescopic sections; the body is provided with four fixed belts which are uniformly and symmetrically distributed on the two sides of the body; each fixed belt is provided with a bonding plate A; the upper end of the demagnetization layer and the lower end of the demagnetization layer are provided with bonding plates B. The orthotic device has the function of correcting leg gestures for the female with handicapped legs, and also enables legs of normal female users to keep line of beauty all the time, so that the body shape is more attractive, and the orthotic device is quick and convenient to use and has higher market value.
Owner:ANHUI UNIV OF SCI & TECH
Method for detecting ovarian cancer
A method of detecting ovarian cancer in a female test subject comprising determining the amount of plasmenyl-PA or a biomarker having a mass charge ratio of approximately 655.3 in a sample of a bodily fluid taken from the female test subject and comparing the amount of plasmenyl-PA (or the biomarker) in the sample of the bodily fluid taken from the female test subject to a range of amounts of plasmenyl-PA (or the biomarker) found in samples of bodily fluids taken from a group of normal female subjects of the same species as the female test subject and lacking ovarian cancer, whereby a lower amount of the plasmenyl-PA (or the biomarker) in the sample of the bodily fluid taken from the female test subject indicates the presence of ovarian cancer.
Owner:THE CLEVELAND CLINIC FOUND +1
Vaginal speculum capable of conveniently fixing abortion suction tube
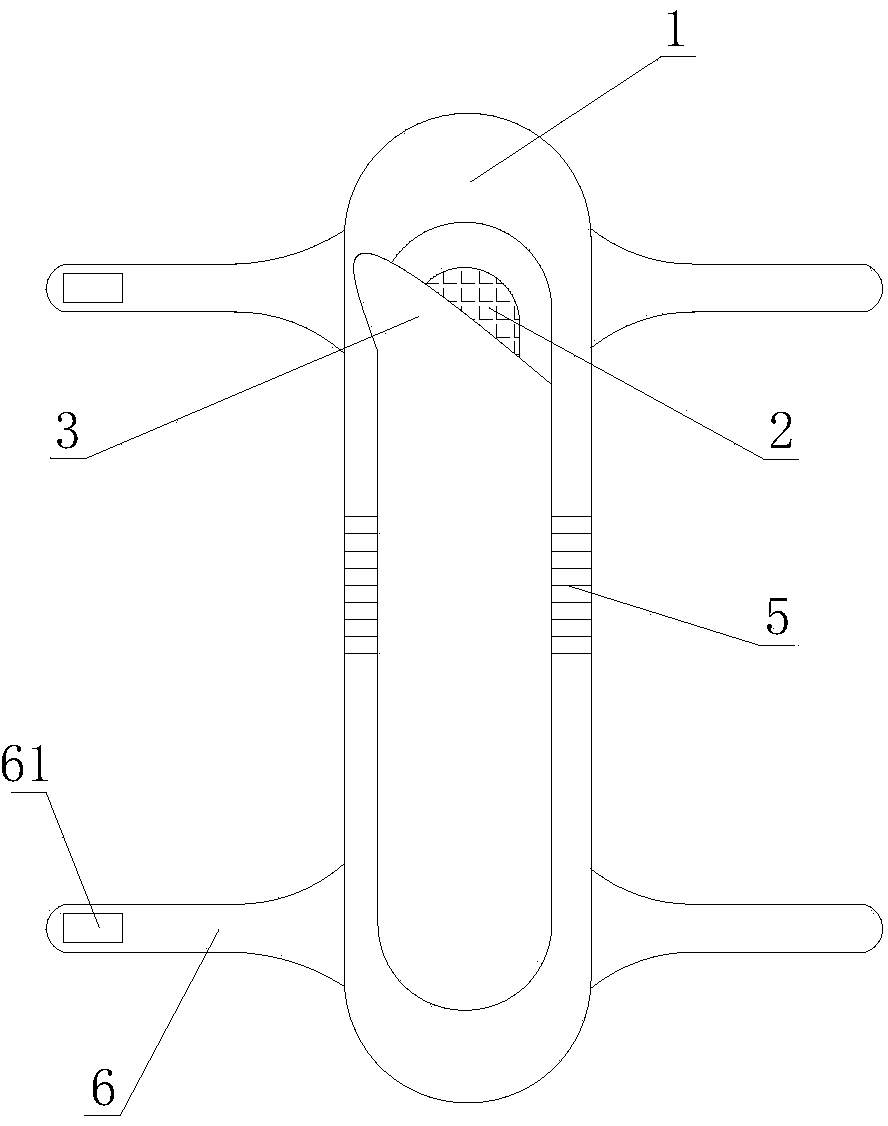
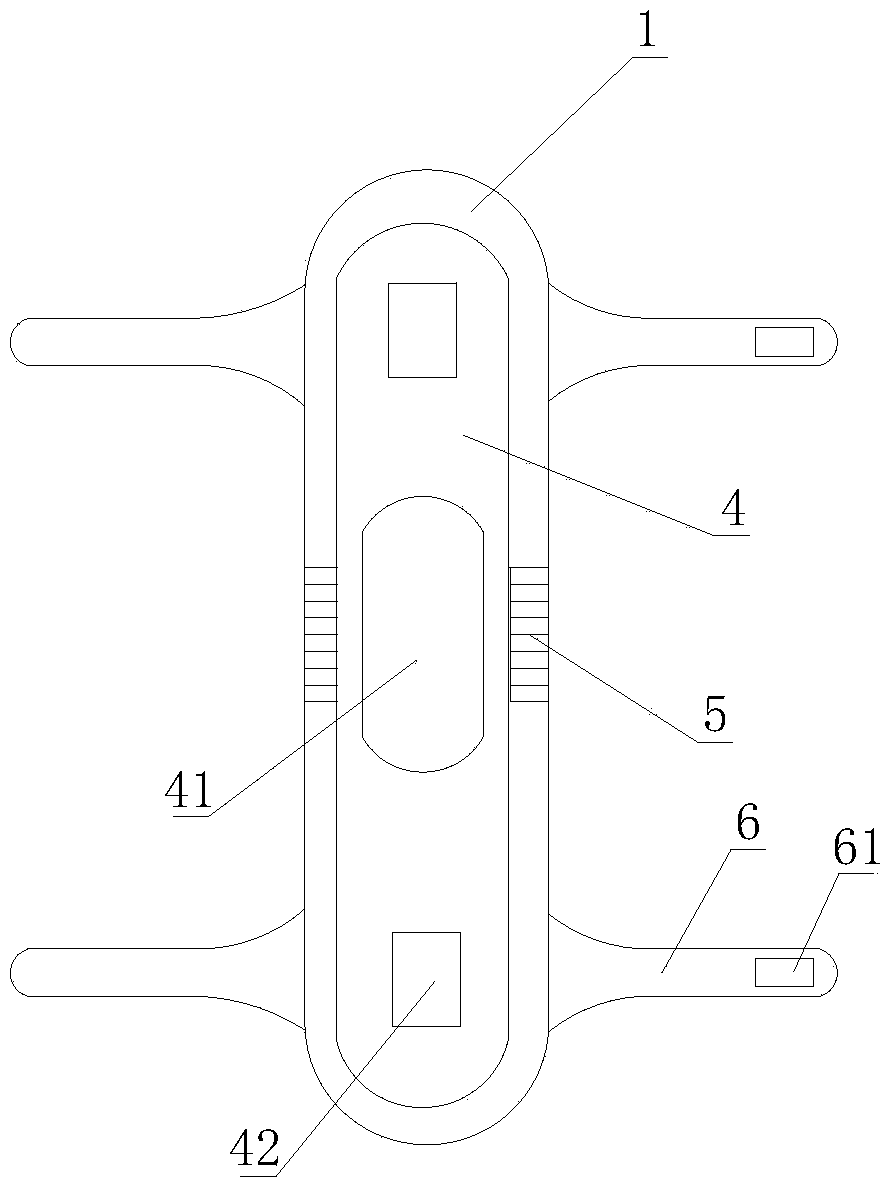
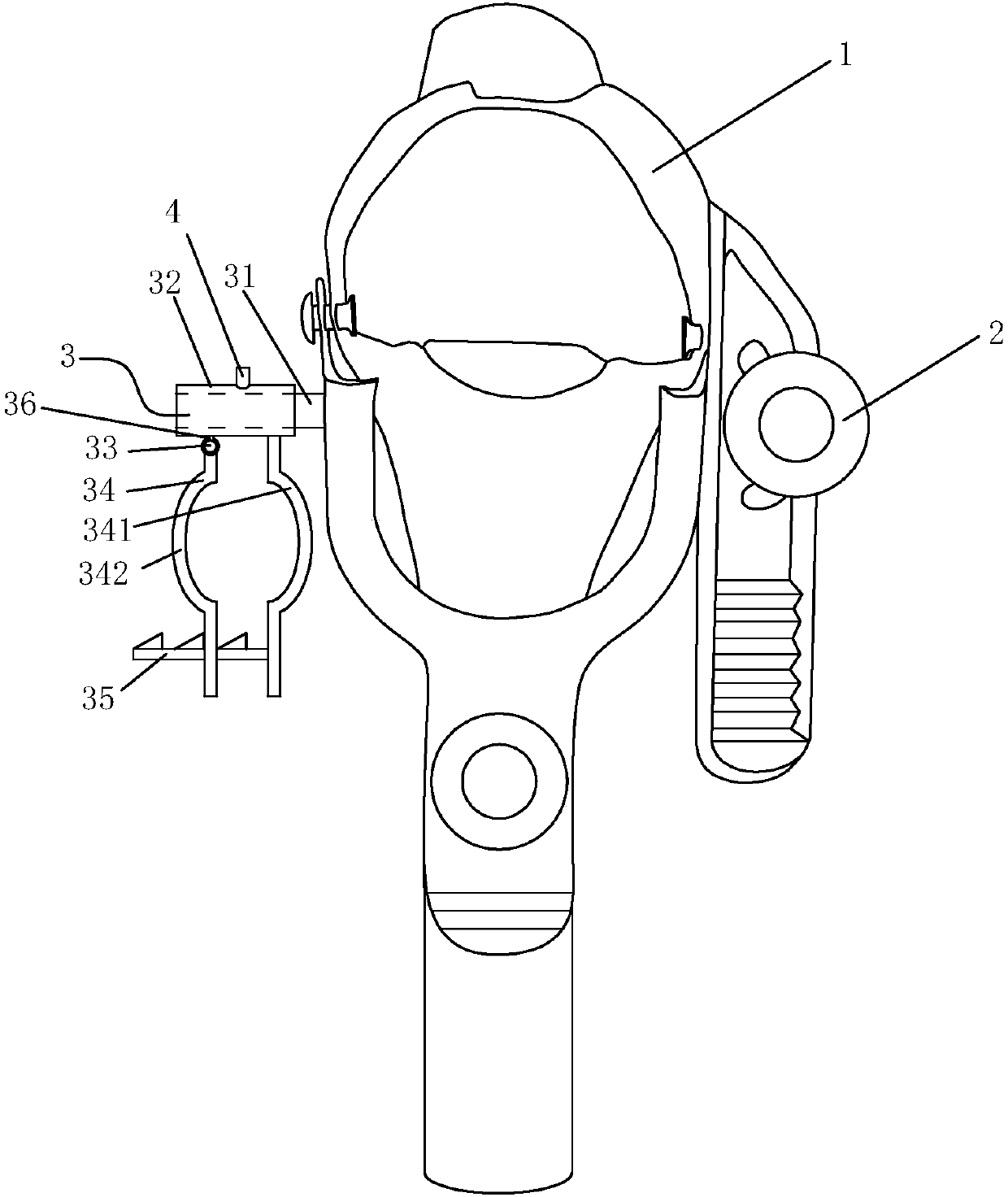
PendingCN107713975AEasy to fixEasy to implementObstetrical instrumentsVaginoscopesNormal femaleAbortion
The invention relates to a vaginal speculum capable of conveniently fixing an abortion suction tube. The vaginal speculum comprises a vaginal speculum main body, wherein a suction tube fixing device is arranged at one side of the vaginal speculum main body; the suction tube fixing device comprises a mounting rod, a connecting sleeve, a fixing clamp and a limiting rod; one end of the mounting rod is fixed to the vaginal speculum main body; the connecting sleeve sleeves the mounting rod; a mounting shaft is connected to the connecting sleeve by virtue of a connecting rod; the fixing clamp is composed of a fixed half clamp and a movable half clamp; one end of the movable half clamp sleeves the mounting shaft; a locating through hole is kept in the other end of the movable half clamp; the limiting rod is fixed to the fixed half clamp; and the locating through hole of the movable half clamp runs through the limiting rod and is fixed to the limiting rod. The vaginal speculum provided by theinvention, by adding the abortion suction tube fixing device on the basis of an original normal vaginal speculum, can fix the abortion suction tube conveniently; the abortion suction tube can be fixedto the suction tube fixing device when a surgery is paused and other operations are implemented, so that the abortion suction tube is protected from getting contaminated; and the vaginal speculum issafe and labor-saving, and the vaginal speculum has relatively good promotion and application values.
Owner:LIUZHOU CITY HEALTHCARE HOSPITAL FOR WOMEN & CHILDREN
Moderation of crustacean androgenic gland peptide for mono sex culture
InactiveUS20160066549A9Induces silencingAlters sexual characteristicAnimal reproductionClimate change adaptationPresent methodChange sex
This invention is directed to methods for manipulating sex, reversing sex, changing sex ratio, or moderating crustacean androgenic gland peptide expression in crustaceans for producing a monosex culture. In one embodiment, the present method comprises the step of injecting a dsRNA for the gene of IAG into a male crustacean. In another embodiment, the present method comprises the step of injecting a dsRNA for the gene of IAG into a female crustacean that had acquired a spermatophore (a sperm sac) after mating with a male. In another embodiment, the present invention provides a method of producing a monosex culture of crustacean by introducing a recombinant IAG peptide into the female crustacean, thereby obtains male with altered sexual characteristics for mating with a normal female. In one embodiment, the crustacean is a shrimp or lobster.
Owner:CHAN SIU MING
Producing method of plant hybrid
InactiveCN1094723CDoes not significantly affect appearanceDoes not significantly affect yieldPlant genotype modificationNormal femaleFructification
The new method selects first-filial generation and the breed with less contour difference to the female parent as parent of cross breeding, and plants the two lines of hybrid in the space-time with normal female parent fructification to raise the uniformity of first-filial generation and utilize the vigor of second-filial generation.
Owner:湖南杂交小麦研究中心
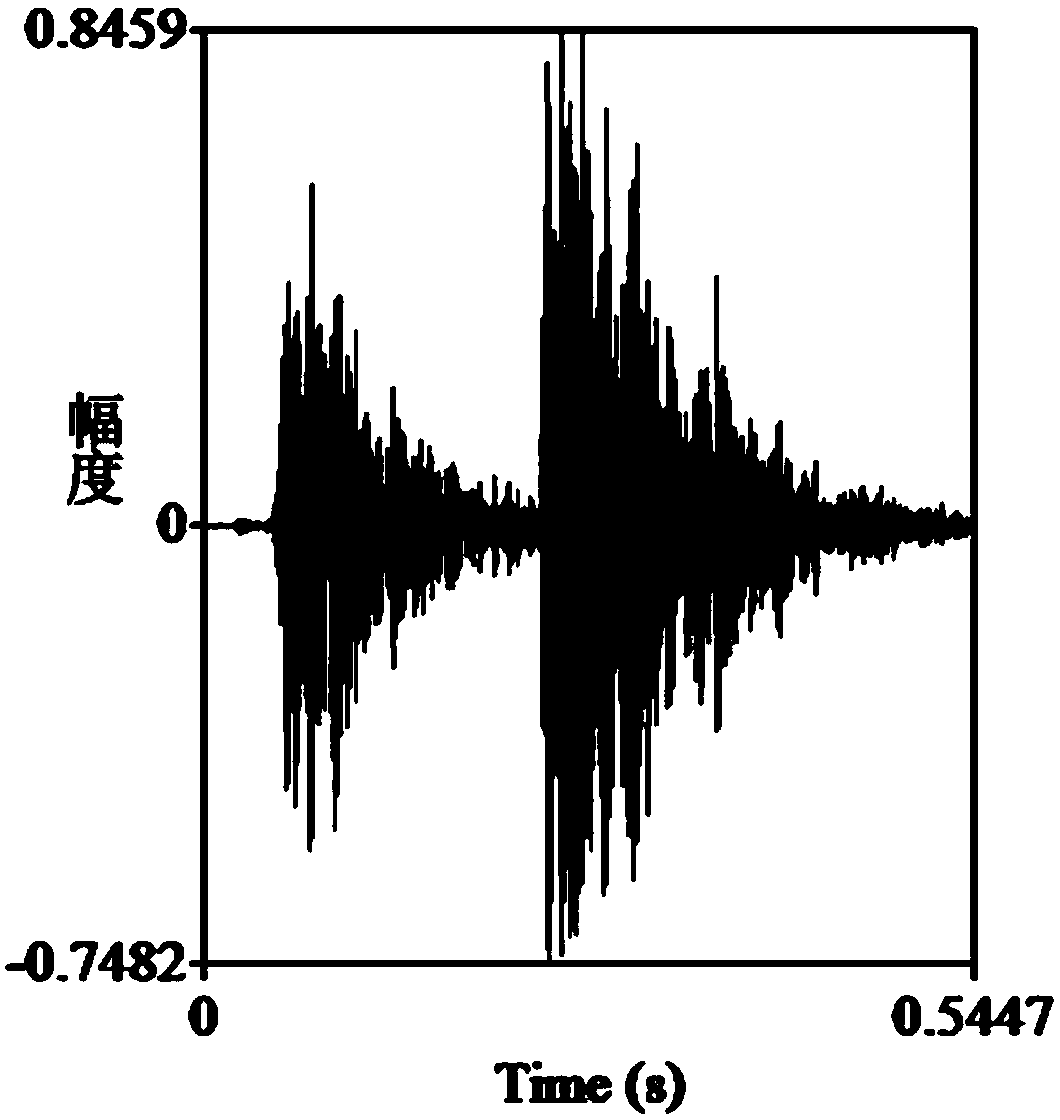
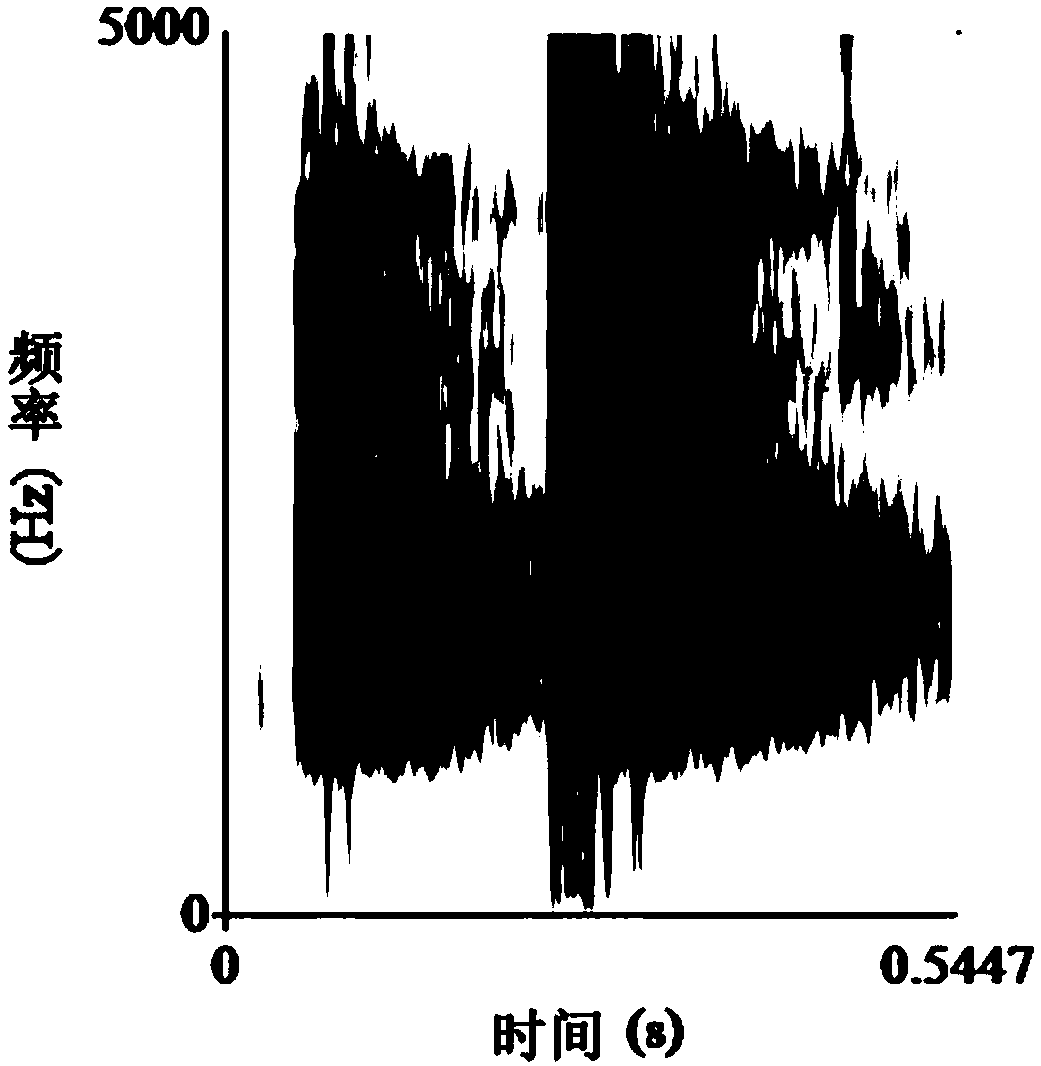
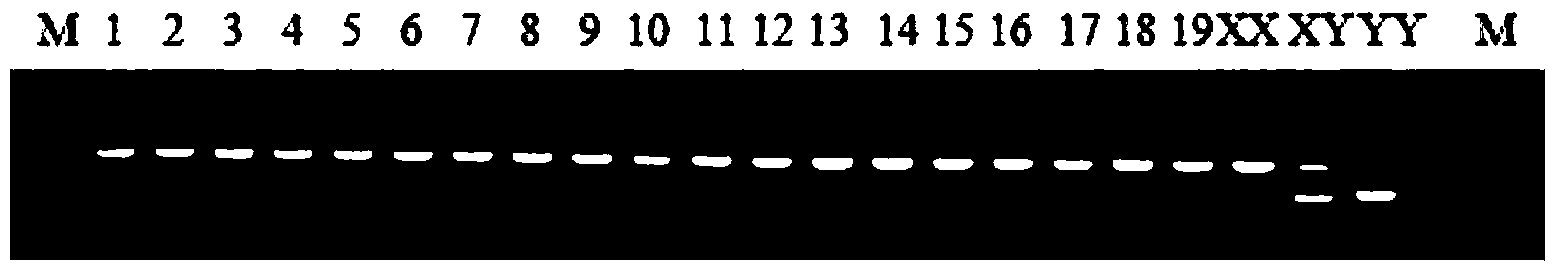
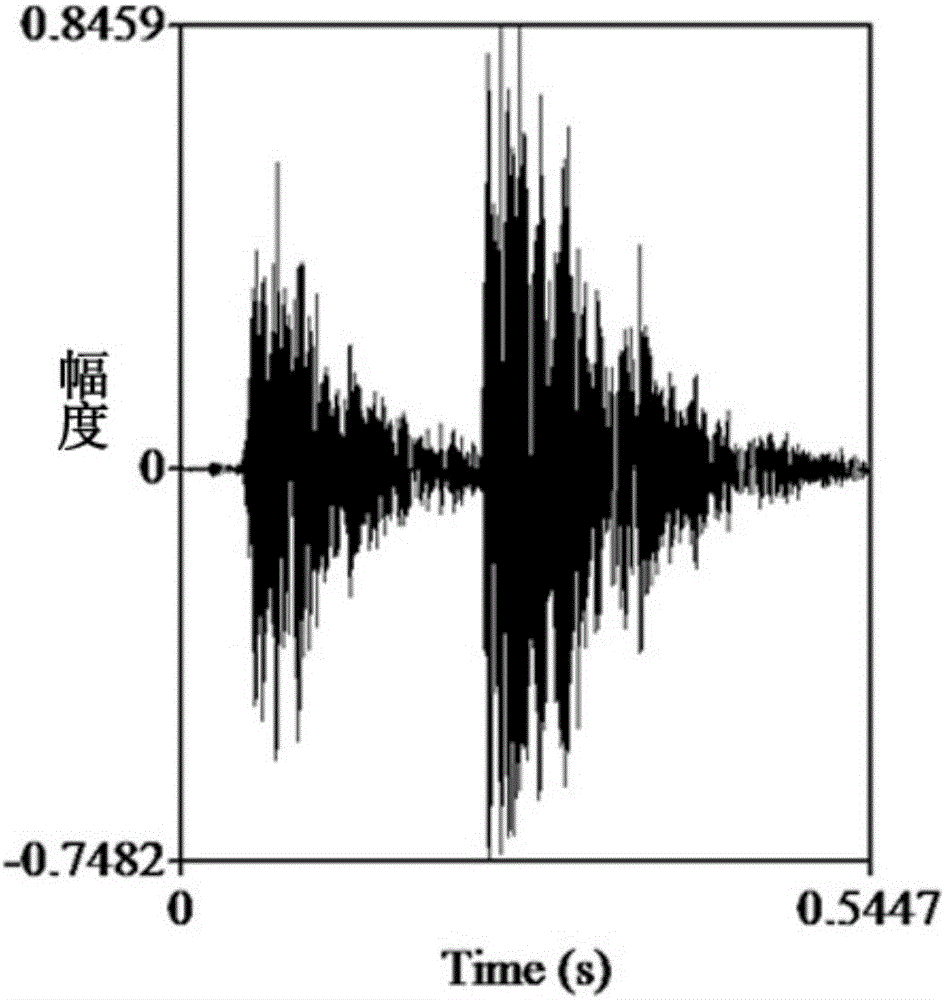
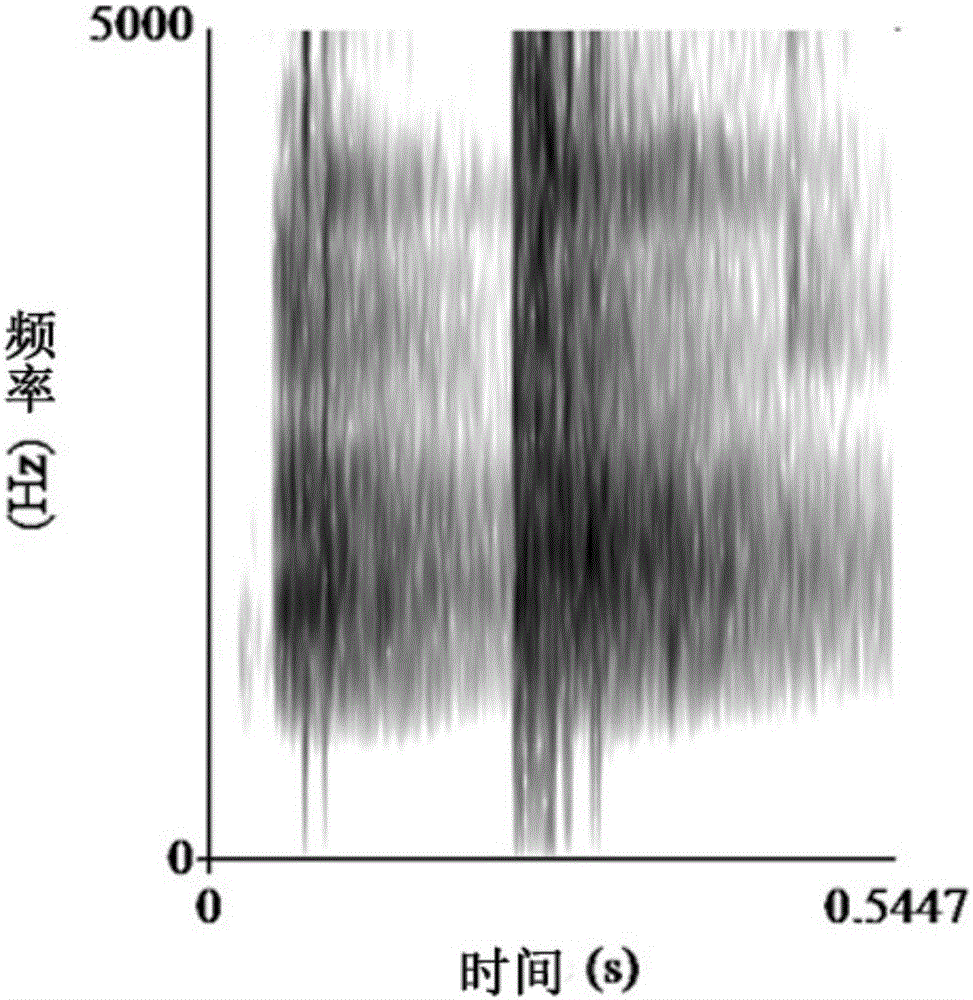
A method for measuring the acoustic communication ability of the black-spotted frog
ActiveCN106070059BEfficiently reflect environmental conditionsClimate change adaptationAnimal husbandryAnimal behaviorRana nigromaculata
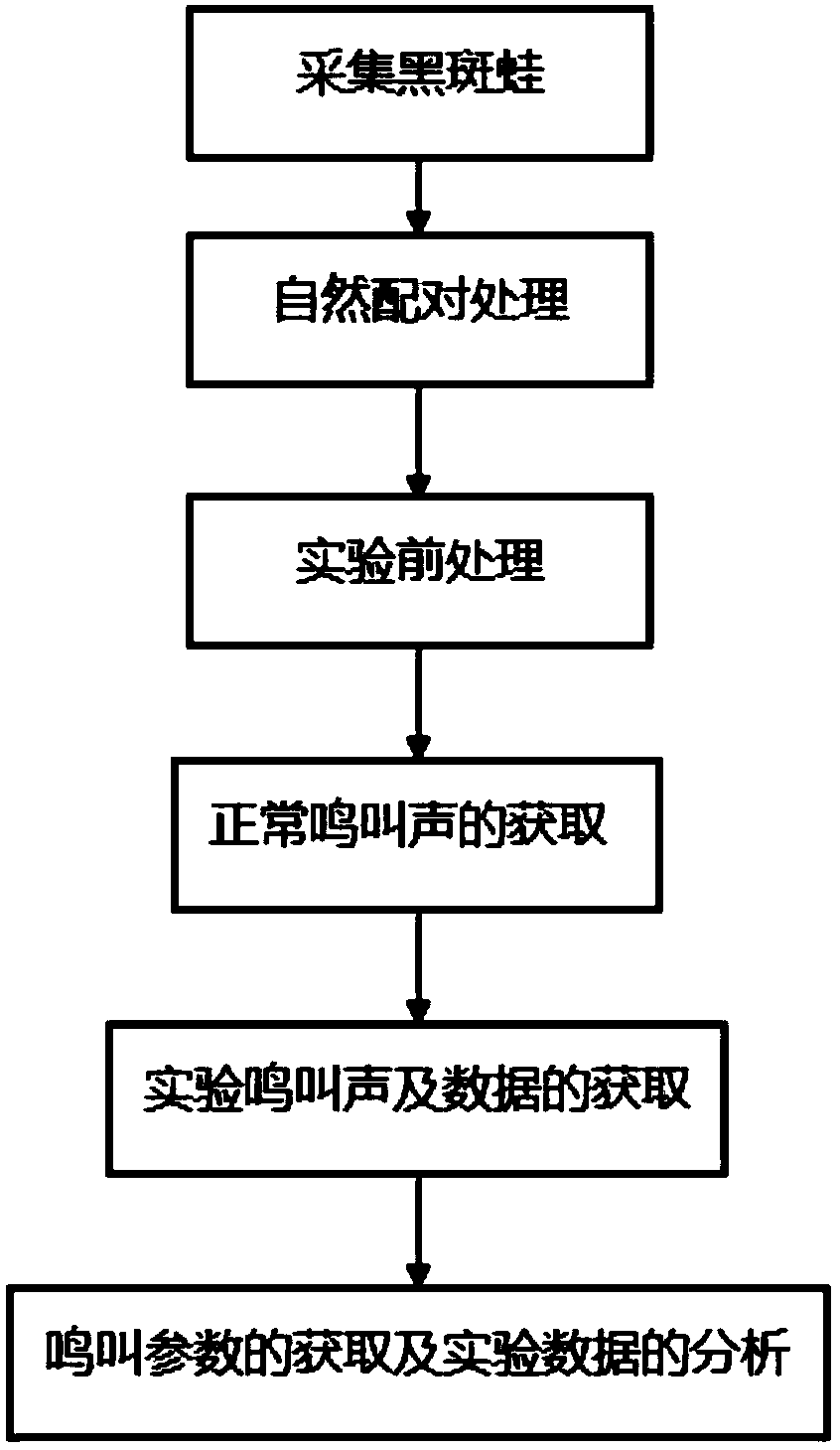
The invention discloses a method for testing the acoustical communication capability of rana nigromaculata. The method comprises the following steps: collecting rana nigromaculata from a natural water body; setting treatment groups and control groups, and performing natural matching treatment on rana nigromaculata; putting all rana nigromaculata in a glass aquarium in a dark room to adapt for 5-15 minutes; recording songs of normal female and male rana nigromaculata in the mating season in advance; playing normal songs of rana nigromaculata, observing response of rana nigromaculata of different treatment groups, and recording corresponding songs and data; and acquiring song parameters, and analyzing experimental data. By adopting the method, important indication significance is provided to further understand the relationship between songs and behaviors of animals and efficiently reflect the body situations and living environment situations of the animals.
Owner:山东巴比熊食品有限公司
Papaya plants having a mutant allele for hermaphroditism
Papaya plants containing mutant allele EWSMHP, which confers production of highly hermaphroditic progenies upon selfing of its hermaphrodite plants and also production of highly hermaphroditic F1 progenies when crossed with normal female and normal hermaphrodite papaya plants, are disclosed. The invention relates to the seeds of papaya plants having mutant allele EWSMHP, to the plants and plant parts of papaya plants having mutant allele EWSMHP and to methods for producing progeny of papaya plants having mutant allele EWSMHP. The invention also relates to methods for producing a papaya plant having mutant allele EWSMHP containing in its genetic material one or more transgenes and to the transgenic papaya plants and plant parts produced by those methods. The invention also relates to papaya cultivars or breeding cultivars, and plant parts derived from papaya plants having mutant allele EWSMHP. The invention further relates to hybrid papaya seeds, plants, and plant parts produced by crossing a plant having mutant allele EWSMHP with another papaya cultivar.
Owner:HORTIGENETICS RES S E ASIA
Traditional Chinese medicine for treating external genital coldness
InactiveCN107397951ARevitalizationSecretion returned to normalSexual disorderPlant ingredientsAngelica Sinensis RootRadix Ophiopogonis
The invention relates to the technical field of traditional Chinese herbal medicines, in particular to a traditional Chinese medicine for treating external genital coldness, and aims to overcome defects of the prior art and solve the technical problem of providing a traditional Chinese medicine for treating external genital coldness. To solve the technical scheme, the invention adopts the technical scheme that the traditional Chinese medicine for treating external genital coldness contains the following traditional Chinese herbal medicines in parts by mass: 10-15 parts of processed rehmannia, 10-15 parts of pulp of dogwood fruits, 10-15 parts of Chinese yams, 10-15 parts of radix ophiopogonis, 10-15 parts of schisandra chinensis, 10-15 parts of angelica sinensis, 5-10 parts of codonopsis pilosula, 10-15 parts of photiniae folia and 5-8 parts of dried ginger. The traditional Chinese medicine is used for treating external genital coldness, activity and secretion of ovaries can be recovered, normal oestrogen secretion is recovered, a normal female hormone level is recovered, and a normal sexual life is recovered.
Owner:张蓓
X-chromosome specific molecular marker of Nile tilapia
ActiveCN103409421BRealize large-scale productionIncrease productionMicrobiological testing/measurementDNA/RNA fragmentationX chromosomeNucleotide
Owner:SOUTHWEST UNIV
Method for testing acoustical communication capability of rana nigromaculata
ActiveCN106070059AEfficiently reflect environmental conditionsClimate change adaptationPisciculture and aquariaAnimal behaviorRana nigromaculata
The invention discloses a method for testing the acoustical communication capability of rana nigromaculata. The method comprises the following steps: collecting rana nigromaculata from a natural water body; setting treatment groups and control groups, and performing natural matching treatment on rana nigromaculata; putting all rana nigromaculata in a glass aquarium in a dark room to adapt for 5-15 minutes; recording songs of normal female and male rana nigromaculata in the mating season in advance; playing normal songs of rana nigromaculata, observing response of rana nigromaculata of different treatment groups, and recording corresponding songs and data; and acquiring song parameters, and analyzing experimental data. By adopting the method, important indication significance is provided to further understand the relationship between songs and behaviors of animals and efficiently reflect the body situations and living environment situations of the animals.
Owner:山东巴比熊食品有限公司
Y-chromosome specific molecular marker of Nile tilapia, and genetic sex determination method and supermale producing method both based on molecular marker
ActiveCN103409420BEconomic divisionQuick distinctionMicrobiological testing/measurementClimate change adaptationNormal femaleNucleotide
The invention discloses a Y-chromosome specific molecular marker of Nile tilapia, wherein the nucleotide sequence of the Y-chromosome specific molecular marker is as shown in SEQ ID No.4; the Y-chromosome specific molecular marker can be commonly used for a plurality of strains of Nile tilapia; a genetic sex determination method of Nile tilapia is established based on the molecular marker, so that normal female fish XX, normal male fish XY, converted female fish XY, converted female fish YY and supermale fish YY can be differentiated from each other economically, rapidly and accurately; large-scale production of supermale fish fry can be realized through mating of YY supermale fish and the normal female fish XX based on the genetic sex determination method; as a result, the yield of culture is improved remarkably, the culture cost is reduced and the economic benefit is enhanced; besides, a method of producing the YY supermale fish through mating of the supermale fish YY and the converted female fish YY is also established by using the Y-chromosome specific molecular marker.
Owner:SOUTHWEST UNIV
Multi-interface SFP network communication connector and production process thereof
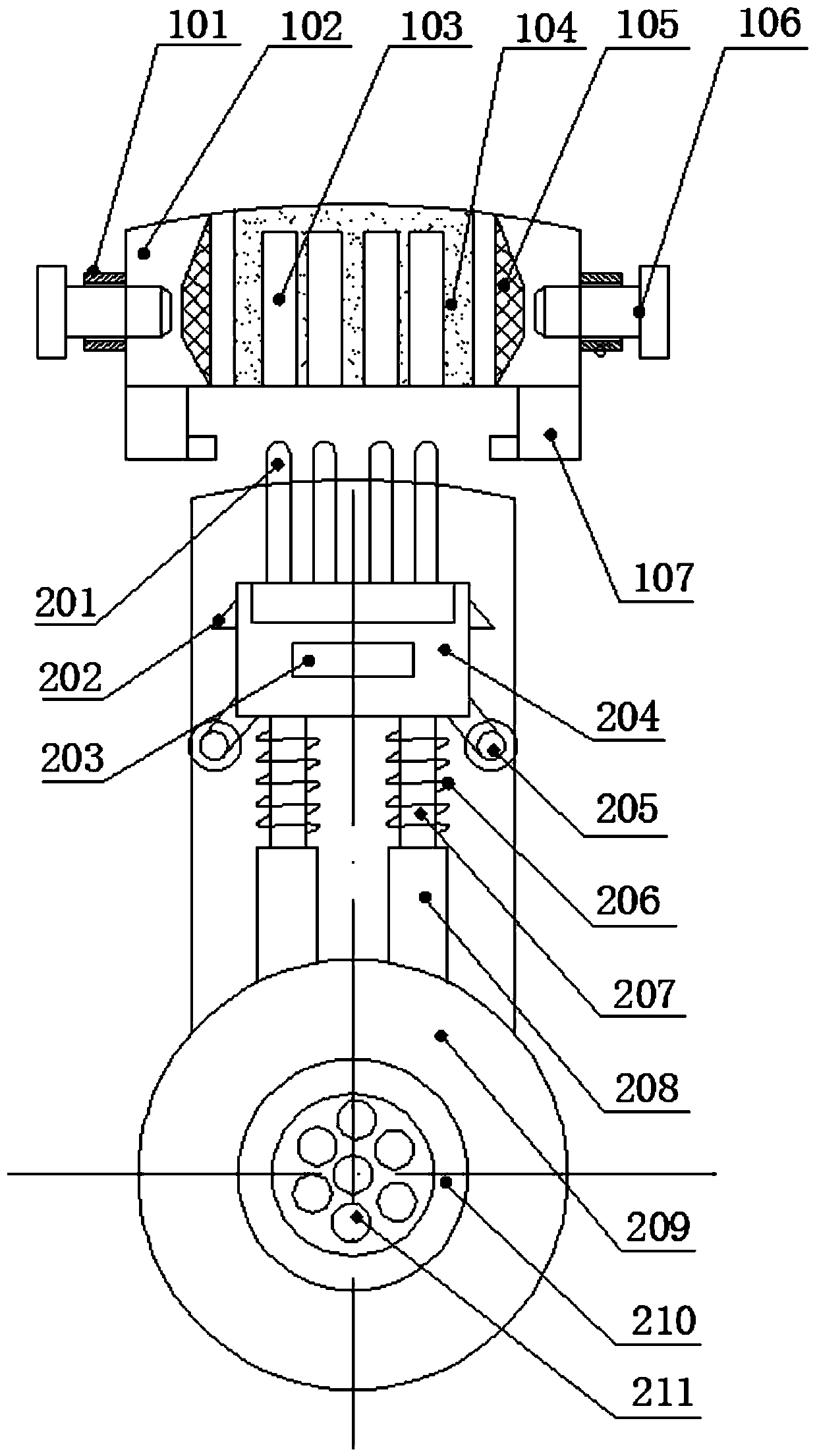
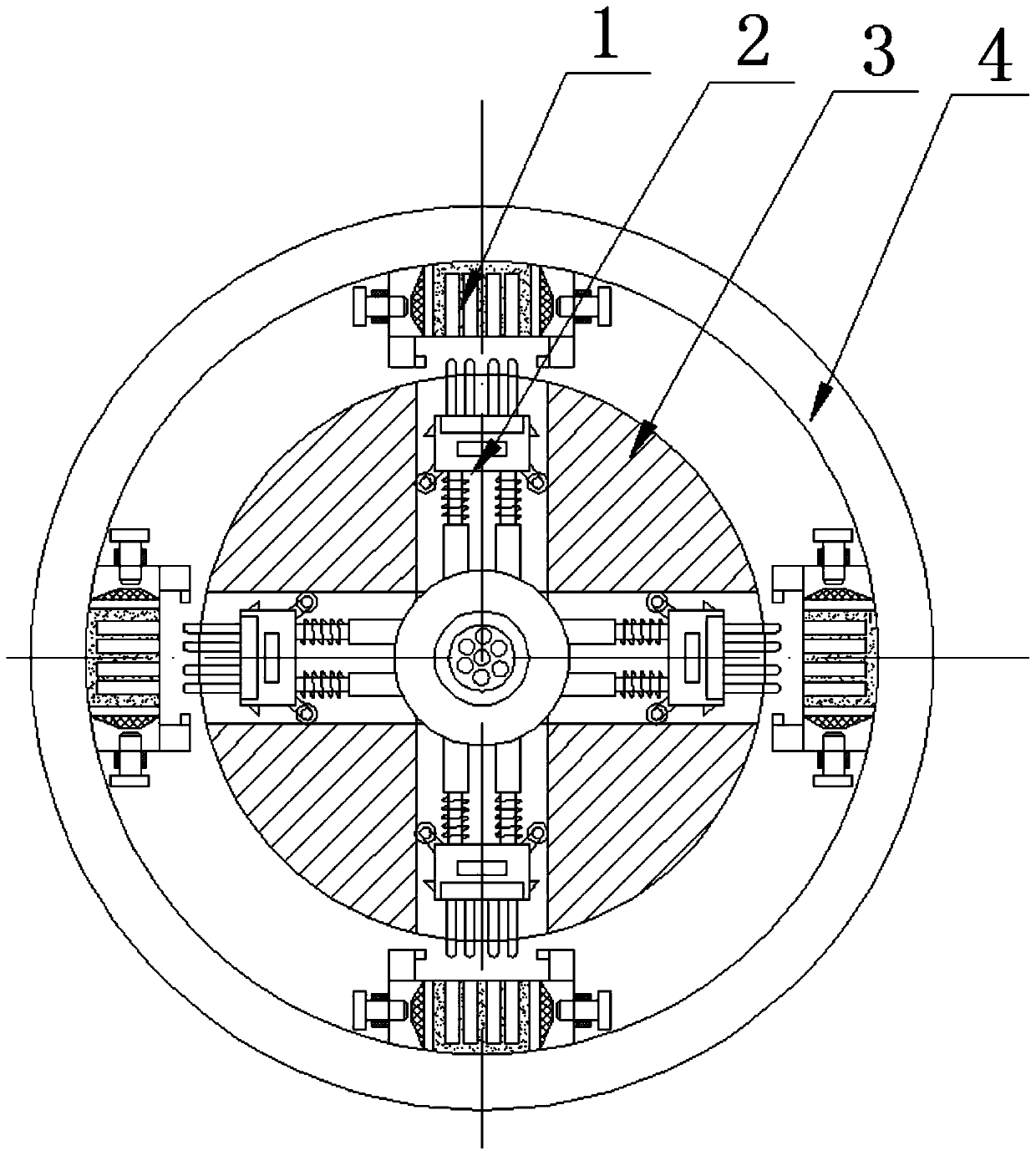
ActiveCN109709647ASimple production processSignal transmission is stableCoupling light guidesNormal femaleNetwork communication
The invention discloses a multi-interface SFP network communication connector and a production process thereof. The connector includes a fixed disk and a rotation disk which is rotatable with an innerring of the fixed disk, wherein periphery of the fixed disk is equipped with multiple annularly-distributed female terminals, and multiple multi-interface type male terminals which can be rotatably switched with the female terminals are mounted around the rotation disk. The connector is advantaged in that in the use process, when one female terminal or male terminal is damaged, switching to the next normal female terminal and male terminal can be performed, the multi-interface type mode is equivalent to a spare mode, when other terminals are damaged, switching rotation is performed to constantly ensure that signal transmission is always stable.
Owner:DONGGUAN DINGTONG PRECISION METAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com