Quality control plasma for noninvasive prenatal detection and preparation method thereof
A technology for prenatal testing and quality control products, applied in biochemical equipment and methods, microbiological measurement/testing, etc., can solve the problem of insufficient positive plasma samples, inability to provide plasma quality control products, and the difficulty in obtaining positive sample plasma and other issues to achieve the effect of alleviating the difficulty of obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] Below in conjunction with embodiment the present invention is described in further detail.
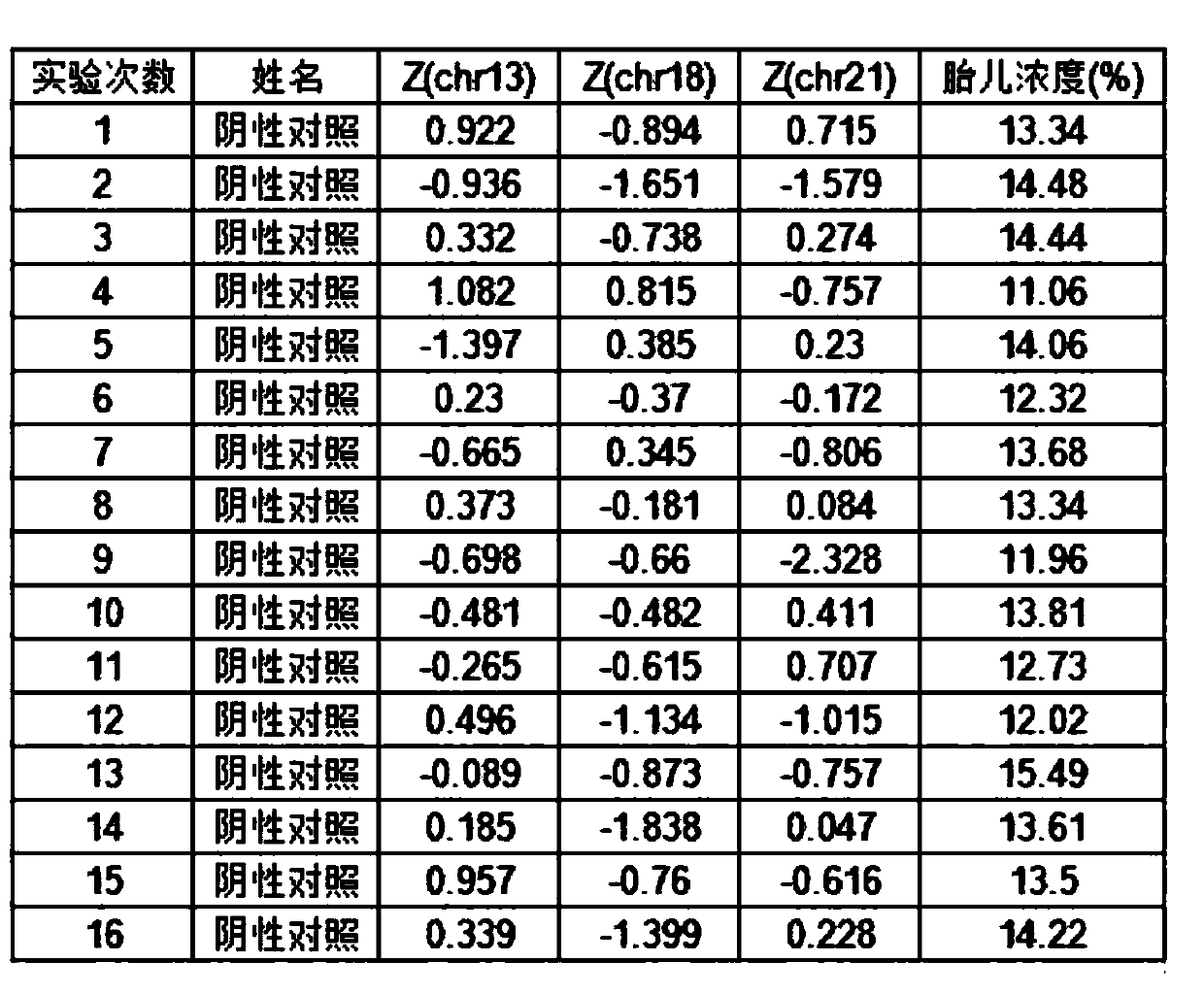
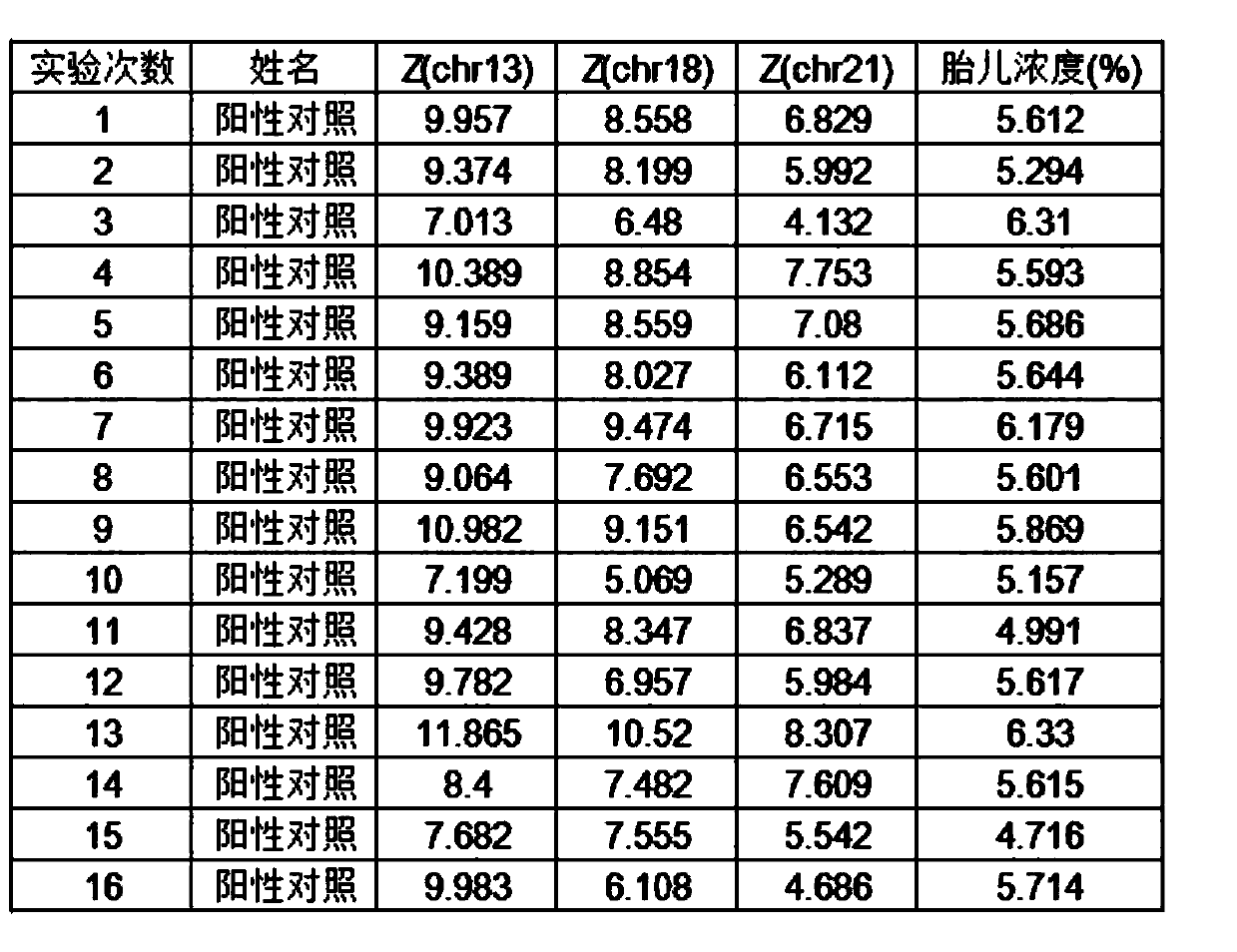
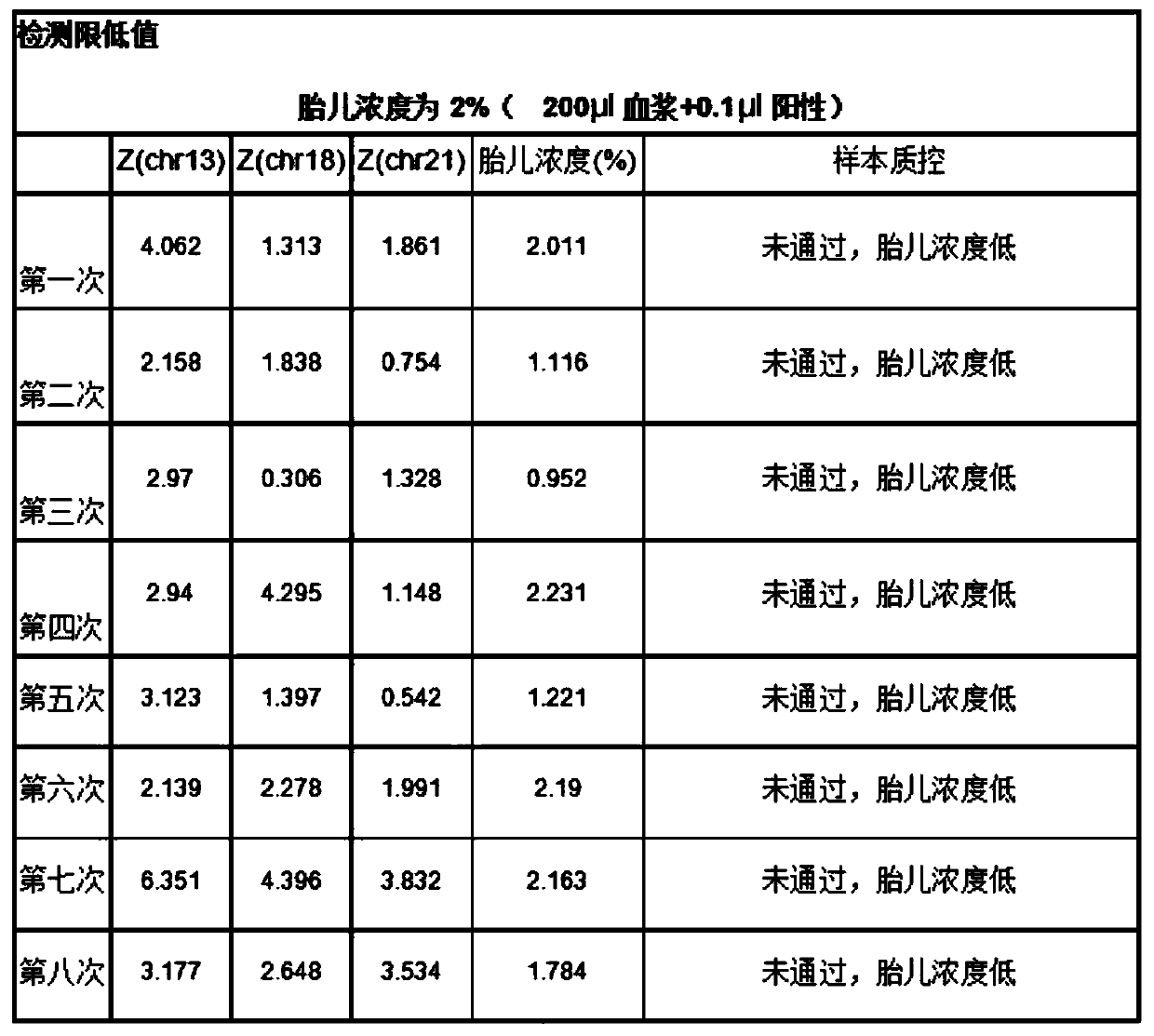
[0030] This example proposes a plasma quality control product for non-invasive prenatal testing, including a positive plasma quality control product, a negative plasma quality control product and a detection limit plasma quality control product. Among them, positive plasma quality control products include a mixture of positive quality control products that come with the verified kit and non-invasive prenatal test results that are low-risk and normal female fetuses after birth. Stored fetal DNA in maternal plasma Concentration between 10-15%; Negative plasma controls include pooled maternal plasma from non-invasive prenatal testing results of low-risk, normal female fetuses after birth; detection limit plasma controls include those from the same healthy non-pregnant female The plasma and the mixed solution of the positive quality control that comes with the validated kit. The re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com