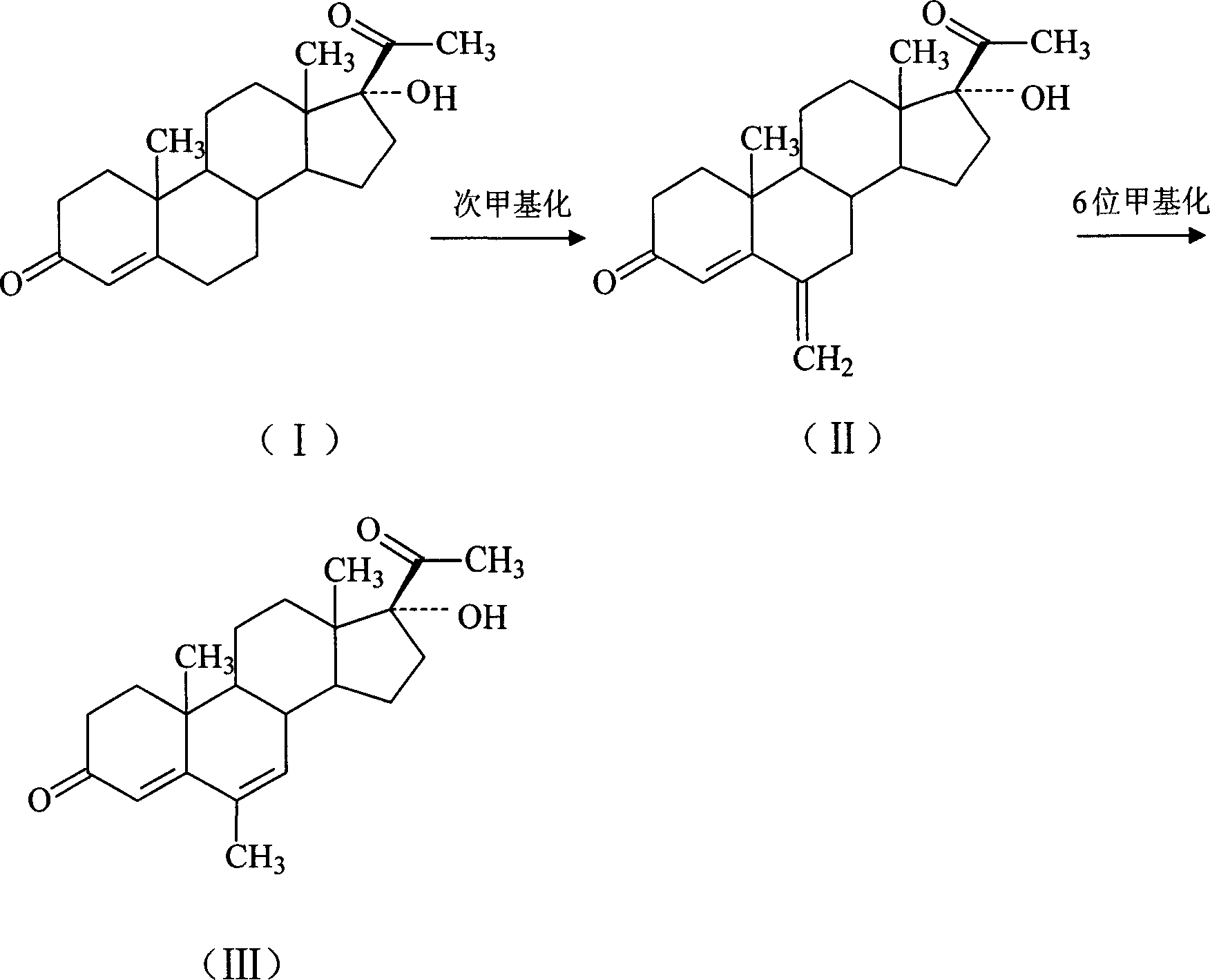
Chemical synthesis of free megestrol
A chemical synthesis, megestrol technology, applied in the direction of organic chemistry, steroids, etc., can solve the problems of incomplete methylation translocation, poor quality of reaction products, long reaction time, etc., to achieve easy industrialization, reduce Generation of by-products, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 30KG of 17α-hydroxyprogesterone was subjected to methylation reaction to obtain 23KG of methine. Pump 260L of dimethylformamide into the reaction tank, put in 23KG of methine, 12KG of 5% palladium-carbon catalyst, and 1200ml of cyclohexene, keep warm at 70°C for 1.5 hours, filter, concentrate while filtering, and concentrate the mother liquor The weight is 1.5 times the weight of the input methine, cooled to below 5°C, centrifuged, rinsed with 50°C warm water for 1 hour, and dried to obtain the crude free megestrol, which was purified to obtain 16.1KG with a yield of 70.0%.
Embodiment 2
[0022] 30KG of 17α-hydroxyprogesterone was subjected to methylation reaction to obtain 23KG of methine. Pump 240L of dimethylformamide into the reaction tank, put in 23KG of methine, 12KG of 5% palladium carbon catalyst, and 1200ml of cyclohexene, keep warm at 60°C for 3 hours, filter, concentrate while filtering, and the mother liquor after concentration The weight is 1.5 times the weight of the input methine, cooled to below 5°C, centrifuged, rinsed with 50°C warm water for 0.5 hour, and dried to obtain free crude megestrol, which was purified to 16.6KG with a yield of 72.2%.
Embodiment 3
[0024] 30KG of 17α-hydroxyprogesterone was subjected to methylation reaction to obtain 23KG of methine. Pump 260L of dimethylformamide into the reaction tank, put in 23KG of methine, 12KG of 5% palladium-carbon catalyst, and 1100ml of cyclohexene, keep warm at 55°C for 2.5 hours, filter, concentrate while filtering, and concentrate the mother liquor The weight is 1.5 times the weight of the input methine, cooled to below 5°C, centrifuged, rinsed with 50°C warm water for 0.5 hour, and dried to obtain the crude free megestrol, which was purified to obtain 16.4KG with a yield of 71.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com