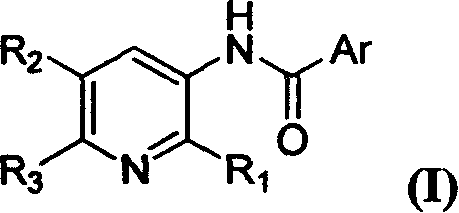
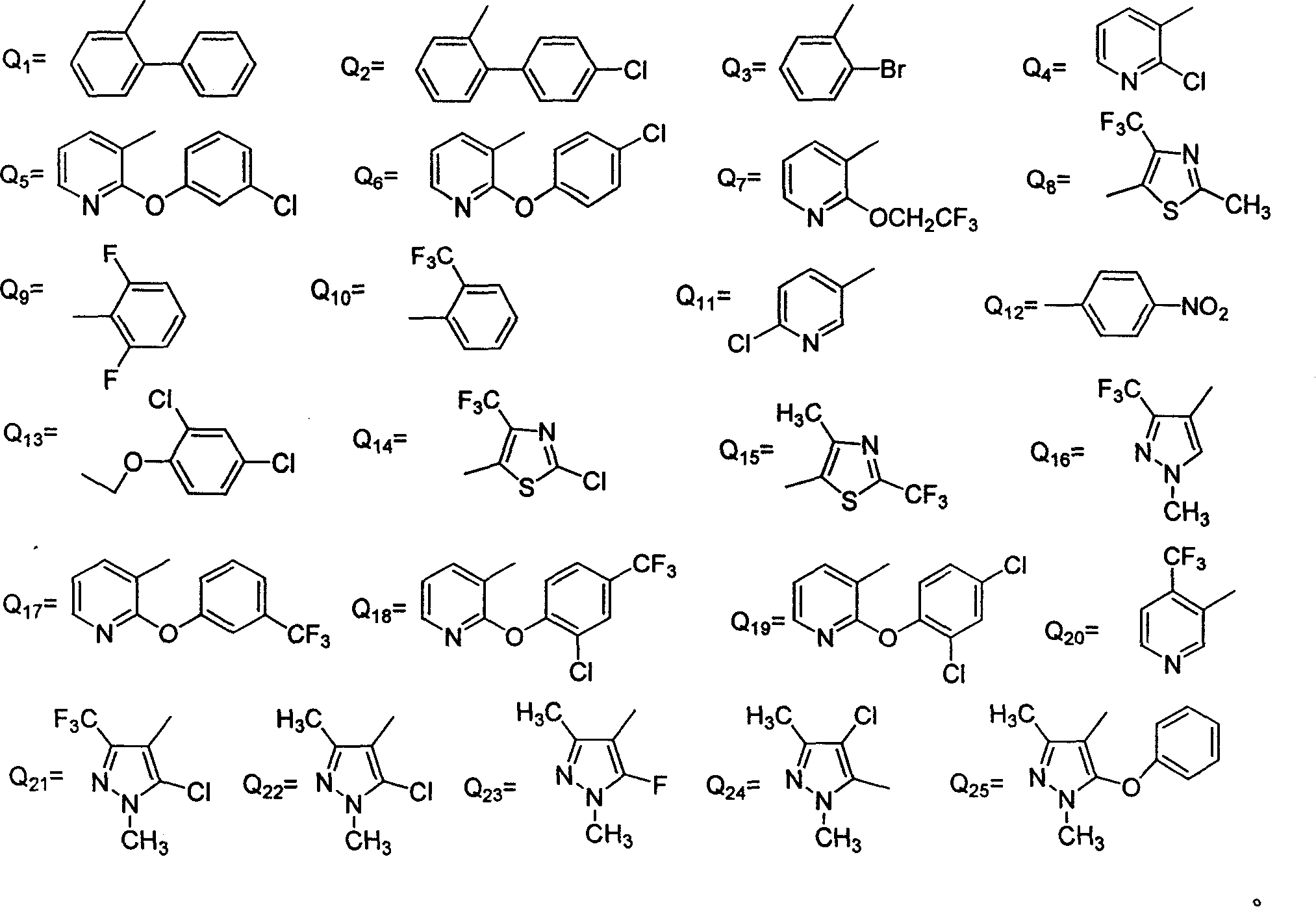
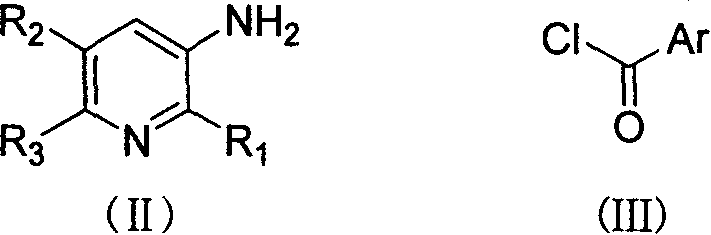Compound of N-(substituted pyridyl)amide, preparation and application thereof
A kind of amide compound and compound technology, which is applied in the field of N-amide compound and its preparation and application, and can solve the problem of no biological activity report and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] Example 1: Preparation of Compound 1
[0057]
[0058] At room temperature, 1.1 g of (III-1) was added dropwise to a solution of 0.6 g of triethylamine and 0.64 g of (II-1) in 20 ml of dichloromethane, and the reaction was stirred for 5 hours. Concentrate the reaction mixture under reduced pressure, add saturated aqueous sodium bicarbonate solution to the residue, extract 3 times with ethyl acetate, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a light yellow solid as crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:4) gave 0.98 g of the title compound with a melting point of 82-84°C. Yield 70.5%.
[0059] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 ) as follows: δppm 7.22 (1H, m), 7.40 (2H, d), 7.45 (3H, m), 7.55 (4H, m), 7.86 (1H, d), 8.02 (1H, d), 8.83 (1H, d).
example 2
[0060] Example 2: Preparation of compound 32
[0061]
[0062]At room temperature, 1.1 g of (III-1) was added dropwise to a solution of 0.6 g of triethylamine and 0.96 g of (II-32) in 20 ml of dichloromethane, and the reaction was stirred for 6 hours. Concentrate the reaction mixture under reduced pressure, add saturated aqueous sodium bicarbonate solution to the residue, extract 3 times with ethyl acetate, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a light yellow solid as crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:4) gave 1.28 g of the title compound, melting at 69-71°C. Yield 68.5%.
[0063] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 ) as follows: δppm 4.61 (2H, m), 6.96 (1H, m), 7.36 (2H, t), 7.42 (3H, m), 7.56 (4H, m), 7.76 (1H, dd), 7.83 (1H, dd), 8.75 (1H,d).
example 3
[0064] Example 3: Preparation of Compound 96
[0065]
[0066] At room temperature, 0.9 g of (III-96) was added dropwise to a solution of 0.6 g of triethylamine and 1.11 g of (II-96) in 20 ml of dichloromethane, and the reaction was stirred for 8 hours. Concentrate the reaction mixture under reduced pressure, add saturated aqueous sodium bicarbonate solution to the residue, extract 3 times with ethyl acetate, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a light yellow solid as crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:3) gave 1.19 g of the title compound, melting at 112-114°C. Yield 66.2%.
[0067] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 ) as follows: δppm 7.12 (1H, dd), 7.18 (2H, d), 7.21 (2H, m), 7.44 (2H, d), 7.58 (1H, m), 7.90 (1H, dd), 8.80 (1H, dd).
[0068] Other compounds were synthesized according to the above me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com