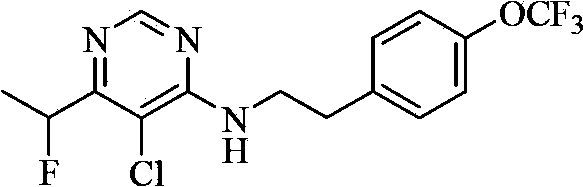
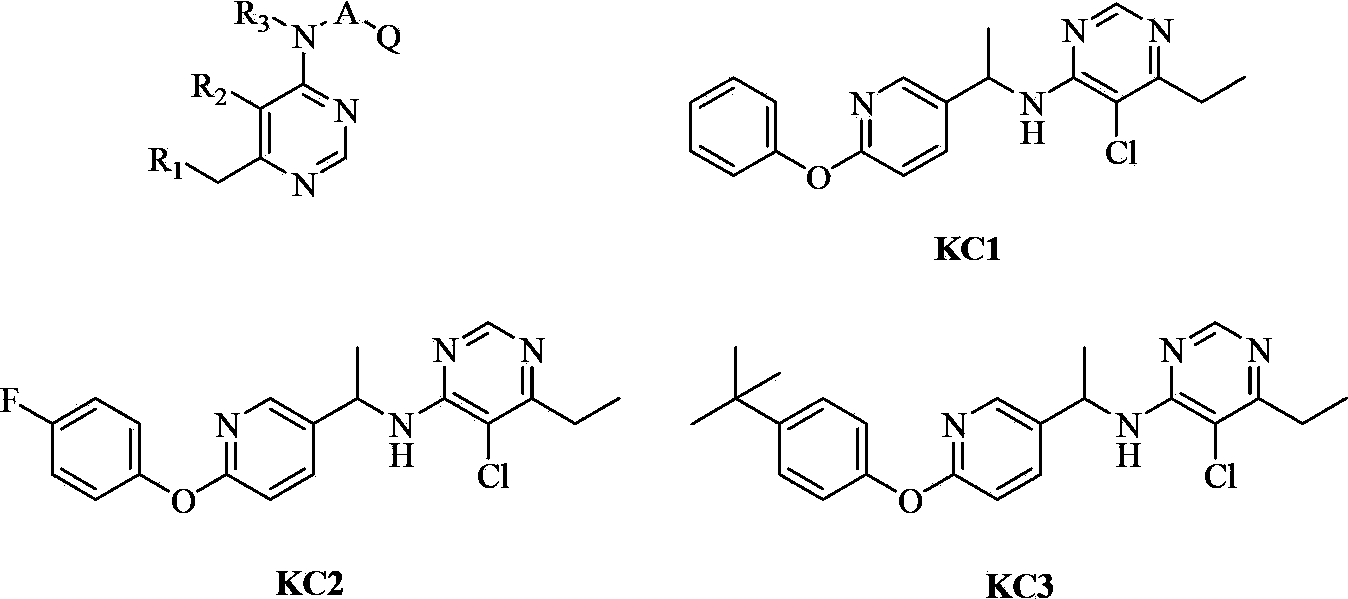
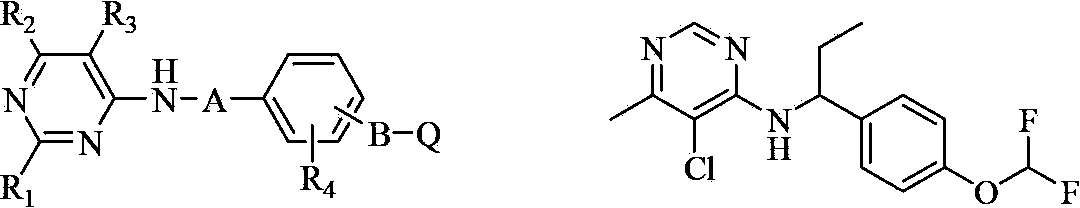Substituted aryloxy pyridine compound and use thereof
A technology for aryloxypyridines and compounds, applied in applications, organic chemistry, biocides, etc., can solve problems such as structural compounds that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0341] Embodiment 1: the preparation of intermediate 4,5-dichloro-6-methylpyrimidine
[0342] 1) Preparation of 4-hydroxy-5-chloro-6-methylpyrimidine
[0343]
[0344] Slowly add 8.80 g (0.16 mol) of sodium methoxide in methanol solution dropwise to 11.30 g (0.11 mol) of formamidine acetate in 50 ml of methanol solution under stirring at room temperature, and continue stirring at room temperature for 2 h after dropping. Then add 11.17g (0.068mol) intermediate ethyl 2-chloroacetoacetate dropwise to the above solution, and continue to stir the reaction at room temperature for 5-7 hours. After the reaction is monitored by TLC, the solvent is evaporated under reduced pressure, and the pH is adjusted to 5 with hydrochloric acid. ~6, suction filtered to get an orange solid, the aqueous phase was extracted with (3×50ml) ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and precipitated. The residue was dissolved in 50ml of ethyl acetate, left overnight, and filtere...
Embodiment 2
[0348] Embodiment 2: Preparation of intermediate 4,5-dichloro-6-difluoromethylpyrimidine
[0349] 1) Preparation of 4-hydroxy-5-chloro-6-difluoromethylpyrimidine
[0350]
[0351] Take 71.9g (0.70mol) of formamidine acetate in a 1000ml three-neck flask, add 150ml of methanol, stir at 5-10°C, and then put the pre-configured and cooled to room temperature 30 % methanol solution of sodium methoxide, pour it into the reaction flask, and then add 100g (0.50mol) of 2-chloro-4,4-difluoroacetoacetate in 100ml of methanol solution to the reaction mixture. The reaction mixture was stirred and reacted for 3-4 hours. After the completion of the reaction as monitored by TLC, the solvent was evaporated under reduced pressure, the pH was adjusted to 5-6 with hydrochloric acid, and 65 g of white solid was obtained by suction filtration. The yield is 73%, and the melting point is 204~206°C.
[0352] 2) Preparation of 4,5-dichloro-6-difluoromethylpyrimidine
[0353]
[0354] Dissolve ...
Embodiment 3
[0355] Embodiment 3: the preparation of intermediate 2-(6-(4-chlorophenoxy)pyridin-3-yl)ethylamine
[0356] 1) Preparation of methyl 6-(4-chlorophenoxy)nicotinate
[0357]
[0358] To 25.6g (0.2mol) of p-chlorophenol in 350mlN, N-dimethylformamide solution, add 103g (3.0mol) of 70% sodium hydride in batches, stir the reaction at room temperature for 4h, then add 34.2 g (0.2mol) methyl 6-chloronicotinate, after the addition is complete, the reaction mixture is heated to 100°C for 10 hours. After the reaction is monitored by TLC, the reaction solution is poured into water, extracted with ethyl acetate, and the organic phase is successively washed with water to saturate Wash with brine, dry, filter, and remove the solvent. After the residue is cooled and solidified, filter, wash with petroleum ether, and dry to obtain 42.0 g of light brown solid, namely methyl 6-(4-chlorophenoxy)nicotinate. Melting point: 64-66°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(pp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com