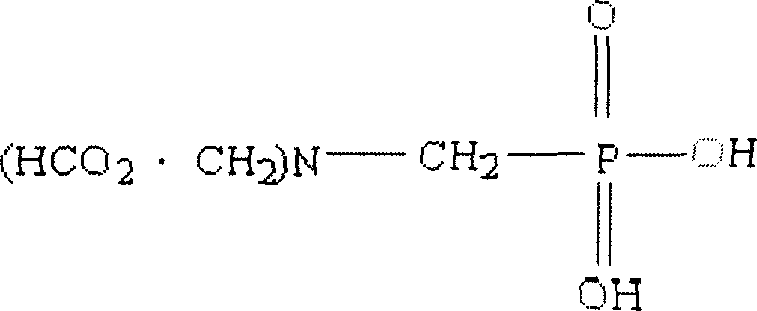Synthetic method for N-phosphonyl methyl imino diacetic acid
A technology of phosphonomethyliminodiacetic acid and iminodiacetonitrile, which is applied in the field of production of N-phosphonomethyliminodiacetic acid, can solve the problems of difficult large-scale production, high production cost, and difficult industrial operation, etc. To achieve the effect of short reaction route and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.1 mol of iminodiacetonitrile, 0.6 mol of sulfuric acid and 0.4 mol of phosphorous acid into a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, raise the temperature to 150°C, add 0.67 mol of 37% formaldehyde, After the addition was completed, the reaction was carried out at 130° C., and the heating was stopped after the complete conversion of iminodiacetonitrile was detected. The temperature was lowered, crystallization was precipitated, filtered by suction, and dried to obtain 86 grams of bisglyphosate with a content of 98.5%.
Embodiment 2
[0025] Add 0.1 mole of iminodiacetonitrile, 0.9 mole of phosphoric acid and 0.8 mole of phosphorous acid into a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, raise the temperature to 80°C, add 1 mole of 37% formaldehyde, After the addition was completed, the reaction was carried out at 90° C., and the heating was stopped after the complete conversion of iminodiacetonitrile was detected. The temperature was lowered, crystals were precipitated, filtered with suction, and dried to obtain 128 grams of bisglyphosate with a content of 98.5%.
Embodiment 3
[0027] Add 0.1 mol of iminodiacetonitrile, 0.3 mol of phosphoric acid and 0.5 mol of phosphorous acid into a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, raise the temperature to 130°C, add 0.56 mol of 37% formaldehyde, After the addition was completed, the reaction was carried out at 150° C., and the heating was stopped after the complete conversion of iminodiacetonitrile was detected. The temperature was lowered, crystals were precipitated, filtered by suction, and dried to obtain 48 grams of bisglyphosate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com