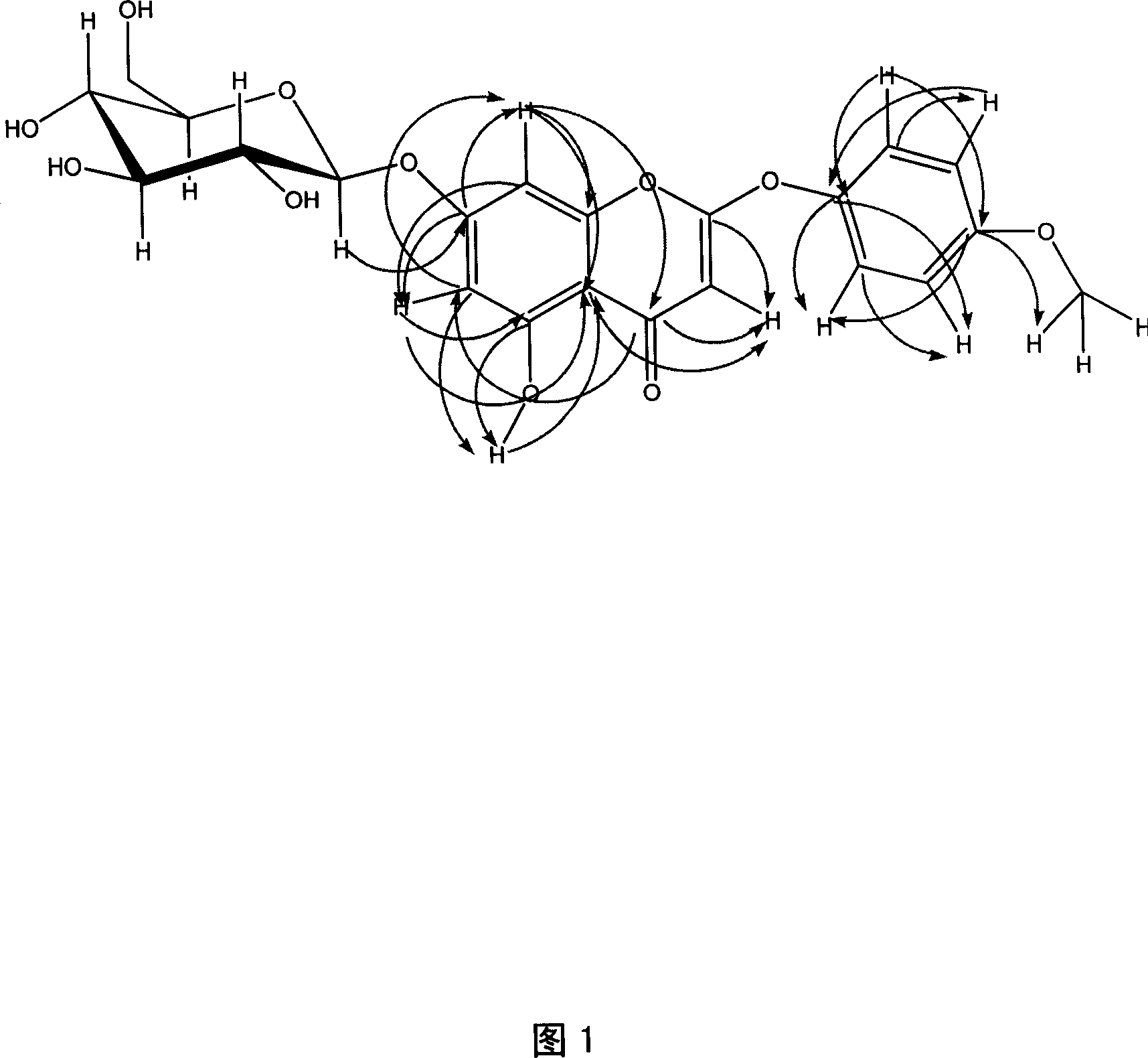
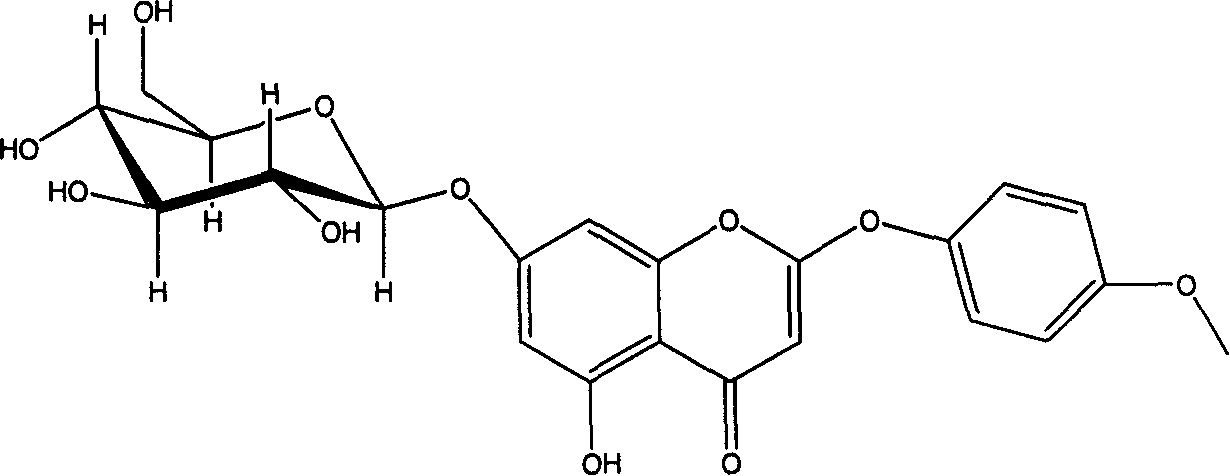
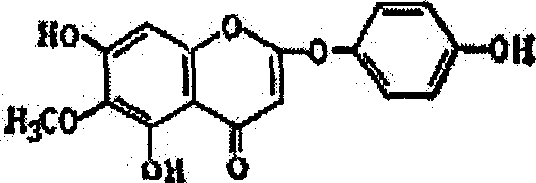
2-phenoxy chromone heteroside compounds and its preparing method and use
A technology of phenoxy chromone and proketone glycosides, which is applied in the preparation of 2-phenoxy chromone glycosides for the treatment of antiviral or immunosuppressant or hyperlipidemia drugs or health care products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Take 2 kg of dried whole Artemisia argyi, heat and reflux with 8 times 60% ethanol solution to obtain the extract;
[0079] Concentrate the extract under reduced pressure at 50°C into a paste, suspend it with 1500ml of water, extract with petroleum ether, ethyl acetate, and n-butanol respectively, recover the n-butanol extract, and concentrate under reduced pressure to extract;
[0080] The extract was dissolved in 500ml of ethanol, mixed with 1 times of silica gel, evaporated the solvent with a rotary evaporator, dried, separated by silica gel column chromatography, packed with 5 times the total sample weight of silica gel column 100 mesh, ethyl acetate: ethanol 100:1 Gradient elution, combined ethyl acetate: ethanol 100: 1 effluent, concentrated and then silica gel column chromatography, ethyl acetate: ethanol gradient elution, silica gel thin layer plate chromatography detection, the same fractions were combined to obtain the The crude compound was finally recrystall...
Embodiment 2
[0082] Take 2 kg of dried whole Artemisia argyi, heat and reflux with 9 times 65% ethanol solution to obtain the extract;
[0083] Concentrate the extract under reduced pressure at 60°C into a paste, suspend it with 1500ml of water, extract with petroleum ether, ethyl acetate, and n-butanol respectively, recover the n-butanol extract, and concentrate under reduced pressure to extract;
[0084] The extract is dissolved in 500ml of ethanol, 1 times of silica gel is used to mix the sample, and the solvent is evaporated with a rotary evaporator, dried, separated by silica gel column chromatography, packed with 150 meshes of silica gel column with 5 times the total weight of the sample, ethyl acetate: ethanol (100: 80) Gradient elution, combined ethyl acetate: ethanol (100: 1) effluent, concentrated and then performed silica gel column chromatography, using ethyl acetate: ethanol gradient elution, silica gel thin layer plate chromatography detection, the same fraction Combined to o...
Embodiment 3
[0086] Take 2 kg of dried whole Artemisia argyi, heat and reflux with 10 times 70% ethanol solution to obtain the extract;
[0087] Concentrate the extract under reduced pressure at 70°C into a paste, suspend it with 1500ml of water, extract with petroleum ether, ethyl acetate, and n-butanol respectively, recover the n-butanol extract, and concentrate under reduced pressure to extract;
[0088] The extract was dissolved in 500ml of ethanol, mixed with 1 times of silica gel, evaporated the solvent with a rotary evaporator, dried, separated by silica gel column chromatography, packed with 5 times the total sample weight of silica gel column 200 mesh, ethyl acetate: ethanol 80:50 Gradient elution, combined ethyl acetate: ethanol 100: 1 effluent, concentrated and then silica gel column chromatography, ethyl acetate: ethanol gradient elution, silica gel thin layer plate chromatography detection, the same fractions were combined to obtain the The crude compound was finally recrystal...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap