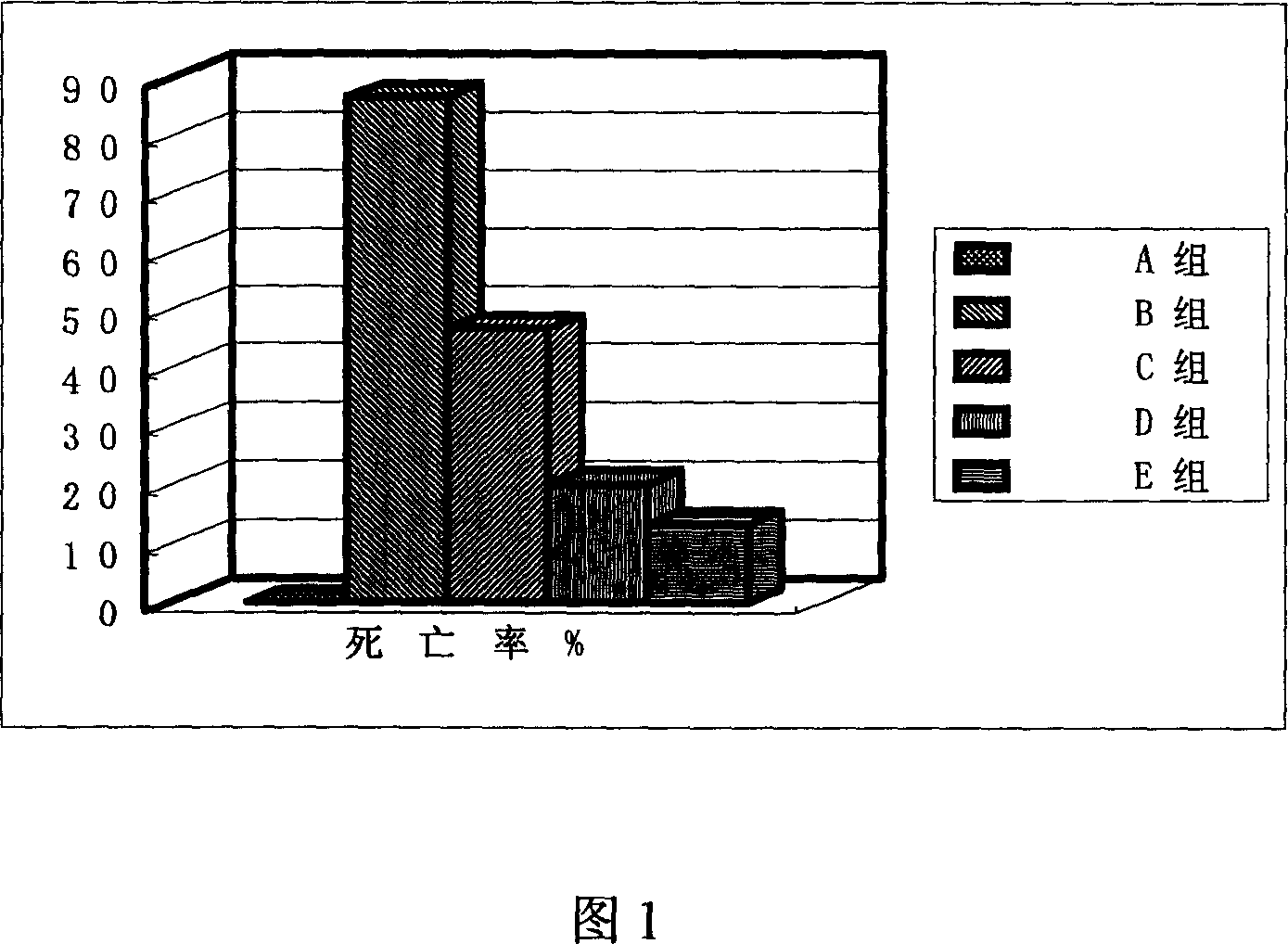
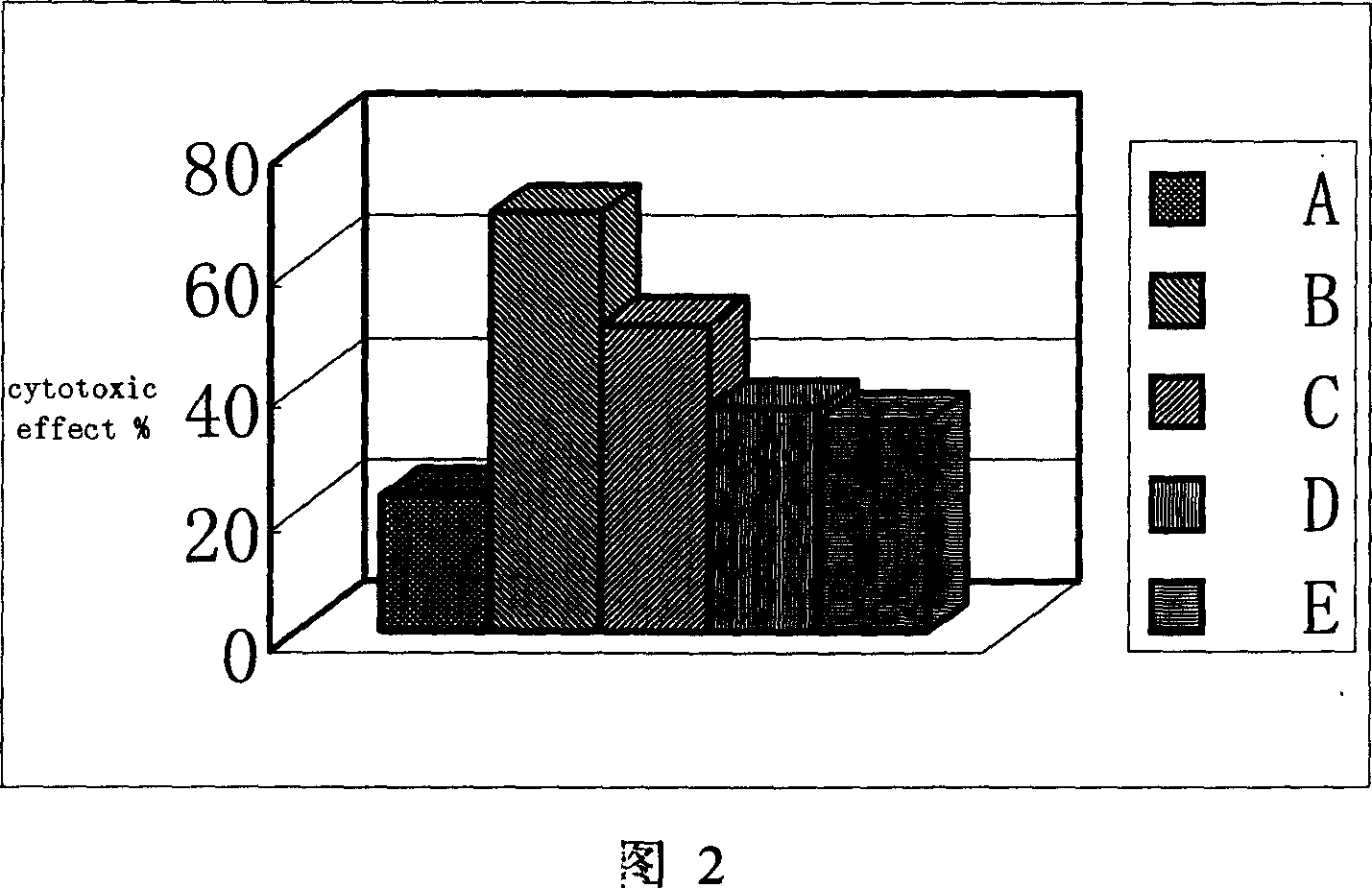
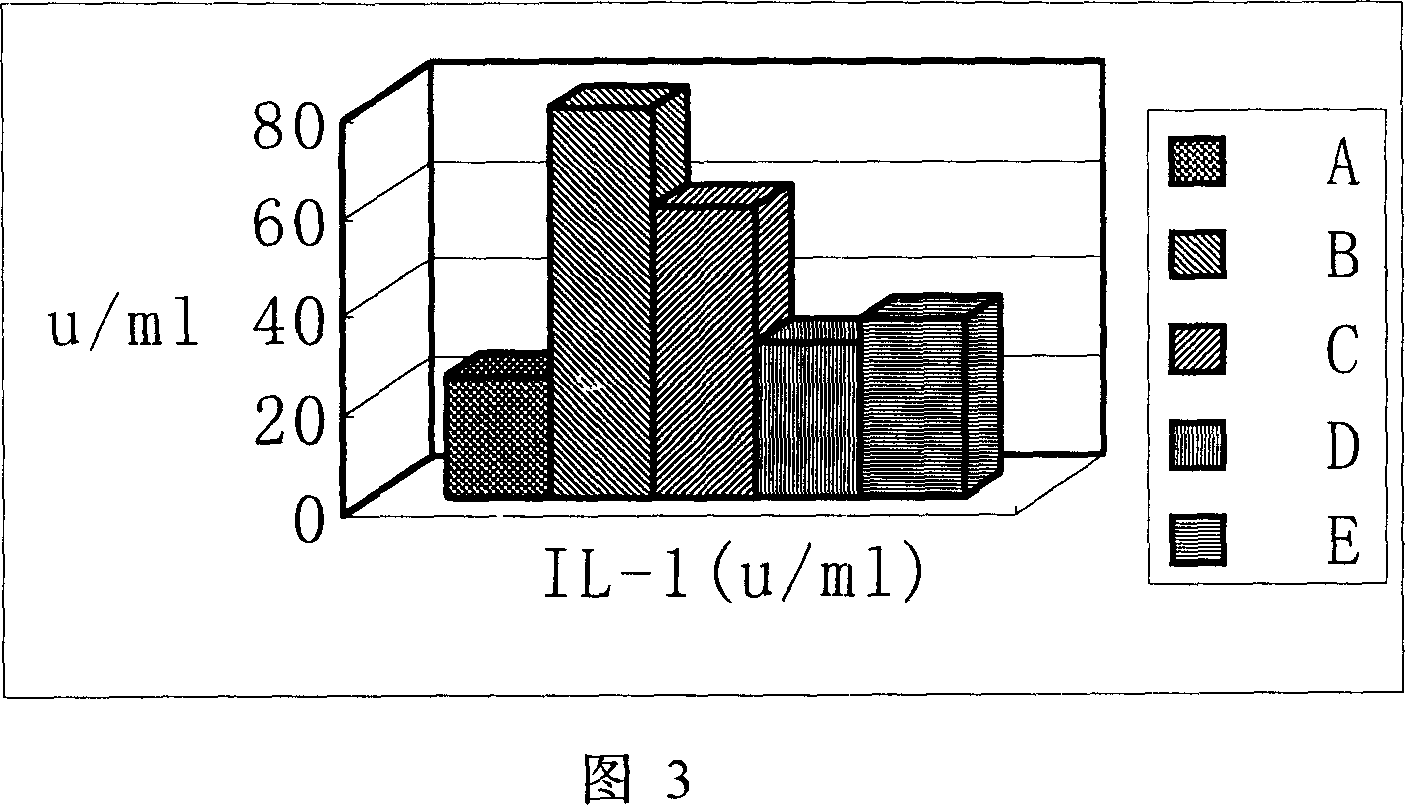
Application of phillyrin in preparation for treating endotoxemia
A technology of endotoxemia and forsythin, which is applied in the field of application of forsythin in the preparation of preparations for the treatment of endotoxemia, and can solve problems that have not yet been developed into a treatment method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Oral enteric-coated capsules:
[0154] Weigh 2 kg of forsythin crude crystals and 60 kg of medical starch, mix well, and put them into No. 5 hollow enteric-coated capsules, so that each capsule contains 4 mg of forsythin coarse crystals, and the total weight of each capsule is 0.12 g , for oral use.
Embodiment 2
[0155] Embodiment 2: intravenous injection:
[0156] Forsythin solution was prepared by dissolving 2mg of refined forsythin in 1ml of 50% pure ethanol; then diluted with water for injection to a total of 10ml, and then filtered three times sequentially, first pre-filtered with F2300G-60 titanium filter , the filtrate is filtered with a nuclear microporous filter membrane (0.45 μm), and finally sterile filtered with a 0.22 μm filter membrane, mechanically potted, and made into an injection with a dose of 2mg / 10mL / ampere for intravenous injection. .
[0157] The purity and concentration of forsythin in the preparation can be determined by ultraviolet spectrophotometry and infrared spectroscopy.
[0158] UV spectrum λ max MeOH (logε): 274;
[0159] Infrared spectrum U max KBr cm -1 : 3380(-OH), 1610, 1598, 1520(C=C)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com