Recombinant virus vector for gene introduction in lymphocyte
A lymphocyte and genome technology, applied in the field of recombinant virus vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1104] (Preparation of recombinant herpes virus)
[1105] (1: Preparation of BAC plasmid)
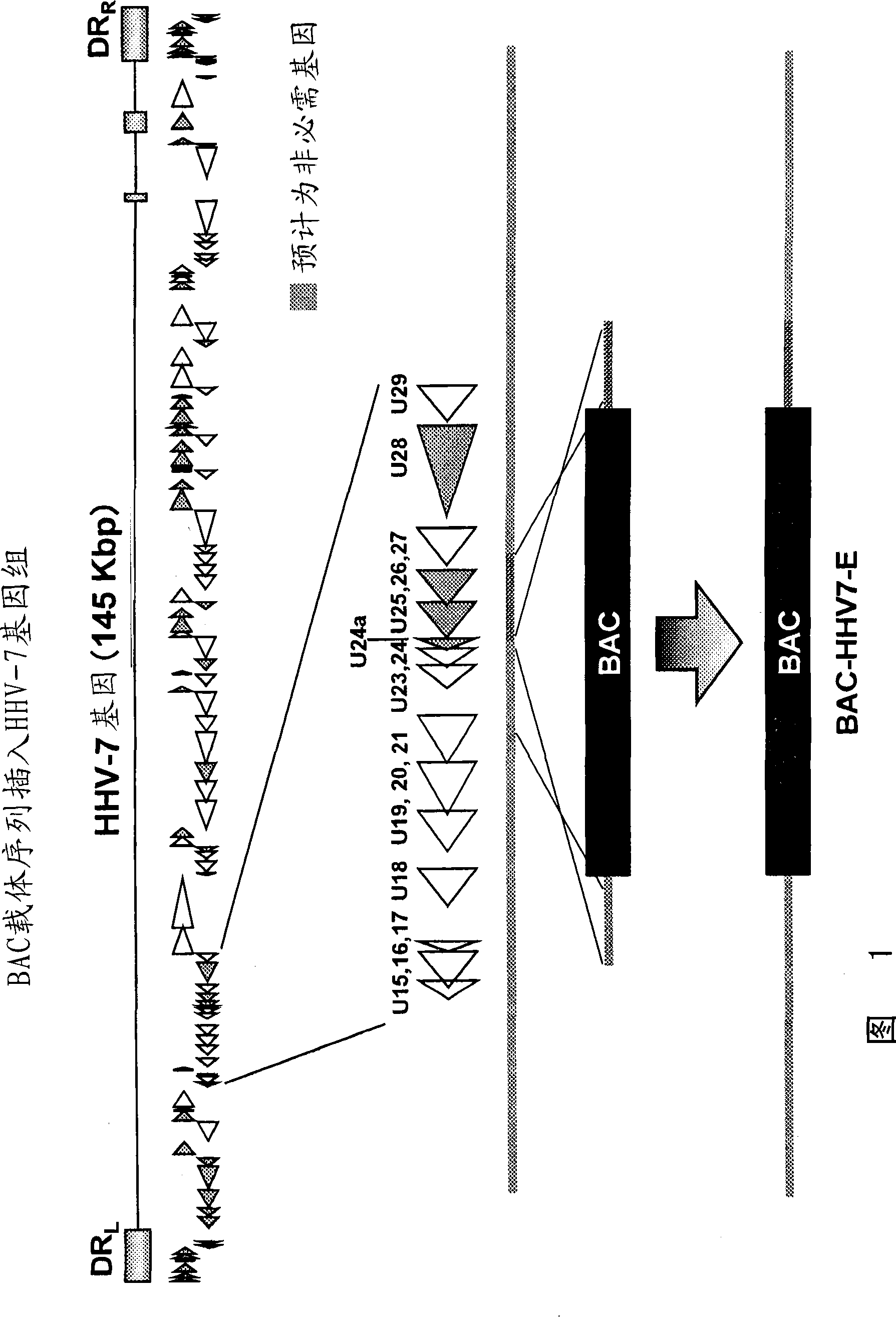
[1106]Plasmid PHA-2, a kind gift from Markus Wagner and Ulrich H. Koszinowski (Adler et al., (2000), J. Virol. 74:6964-74), was used. In order to prepare the recombinant virus, the insertion position of the BAC vector was selected in the center of the region of about 4000 bp of gene U21, gene U23, gene U24, gene U24a, gene U25 and gene U26. This is based on the consideration that insertion of foreign nucleic acid into such non-essential regions does not affect the proliferation of herpes virus. figure 1 is a schematic diagram of a protocol for inserting a BAC vector into the HHV-7 genome.
[1107] Using the genomic DNA of the herpesvirus KHR strain as a template, use primers BAC7-E1 (ATGCGGCCGCGCGAGTGATAGGTACTTTCT) (SEQ ID NO.: 402) and BAC7-E2 (GCTTAATTAATATATAAGTCCTTCAATAGC) (SEQ ID NO.: 403), and primer BAC7-E3 (GCTTAATTAACATGCTCTGCAATGCAAGCC) (SEQ ID NO.: 404) and BAC7-E4 (ATGCGG...
Embodiment 2
[1122] Characteristics of Recombinant Herpes Viruses
[1123] 1. Propagation of Recombinant Viruses
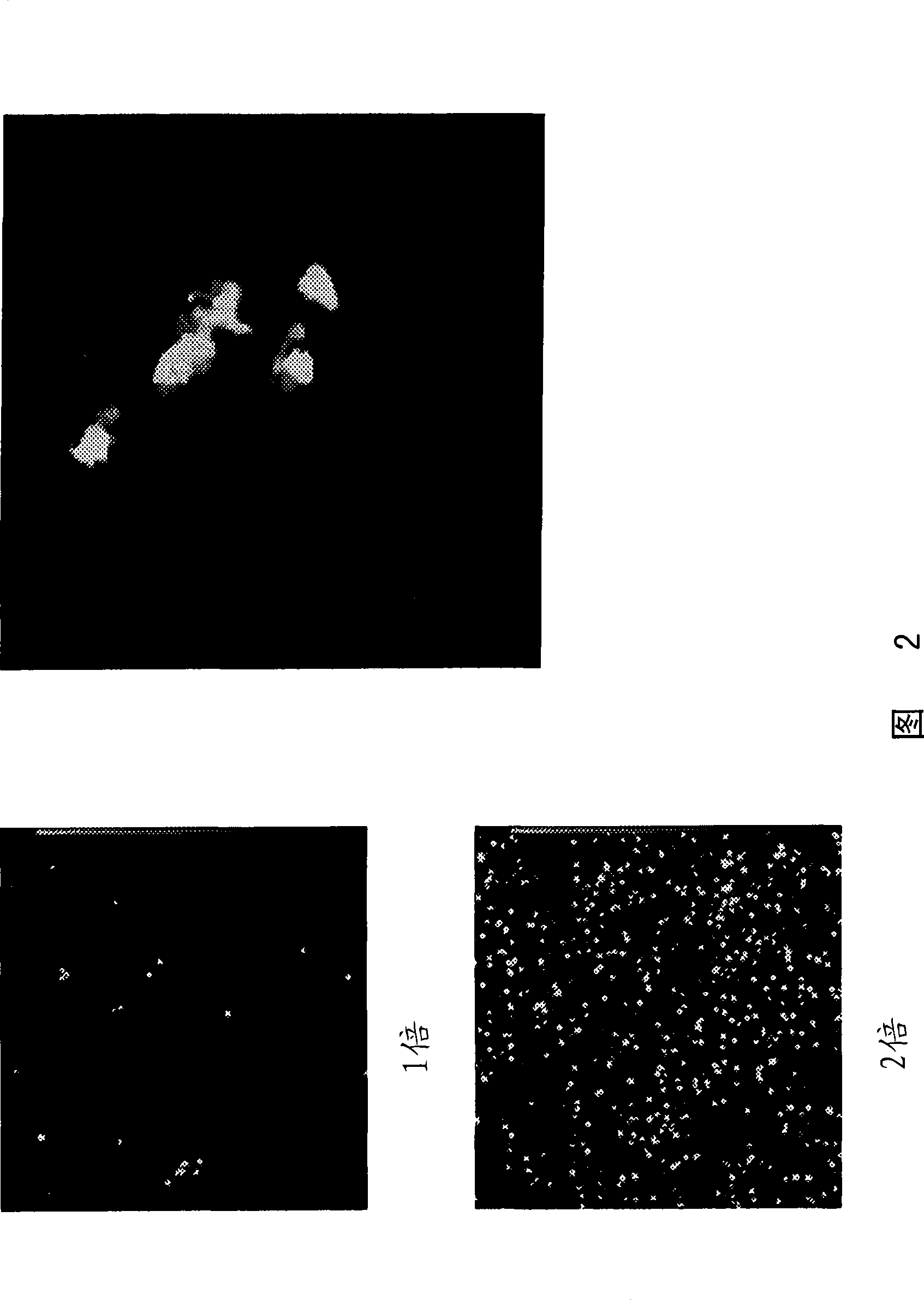
[1124] Propagation of the recombinant herpes virus prepared by the method described in Example 1 was confirmed.
[1125] Umbilical cord blood mononuclear cells were infected with the recombinant virus and cultured until day 7. Then the virus-infected umbilical cord blood mononuclear cells were mixed with SupT1 cells for culture. (3,000rpm, 37°C, 40min centrifugal mixing). When the infected cells were observed under a fluorescence microscope, cells with GFP expression and cytopathic effects could be confirmed. The results indicated that the recombinant herpesviruses of the present invention spread between cells and maintained the ability to proliferate (data not shown).
[1126] 2. Comparison of proliferation of recombinant viruses
[1127] In addition to the following TCID50 method, methods for comparing the proliferative properties of recombinant viruses with wild-type v...
Embodiment 3
[1131] Using the method of the present invention, a herpes virus having a herpes virus genome into which an HIV silencing gene (for example, an antisense nucleic acid against HIV) has been introduced can be easily prepared by the following steps.
[1132] A shuttle vector is prepared, in which the NFκB / Sp1 site of the U3 site of the LTR of HIV is effectively connected to the upstream of the HIV silence gene, and its two ends are connected to the flanking regions of non-essential genes of HHV-7. The shuttle vector and HHV-7U21-27-BAC plasmid (plasmid containing HHV-7 genome and BAC vector sequence) were introduced into Escherichia coli. As a result, in Escherichia coli, homologous recombination occurred between the HHV-7-U21-27-BAC plasmid and the shuttle vector, resulting in the introduction of the foreign gene (HIV silencing gene) contained in the shuttle vector into the HHV-7U21- Cointegrates formed in the 27-BAC plasmid.
[1133] Next, the shuttle plasmid was removed since...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com