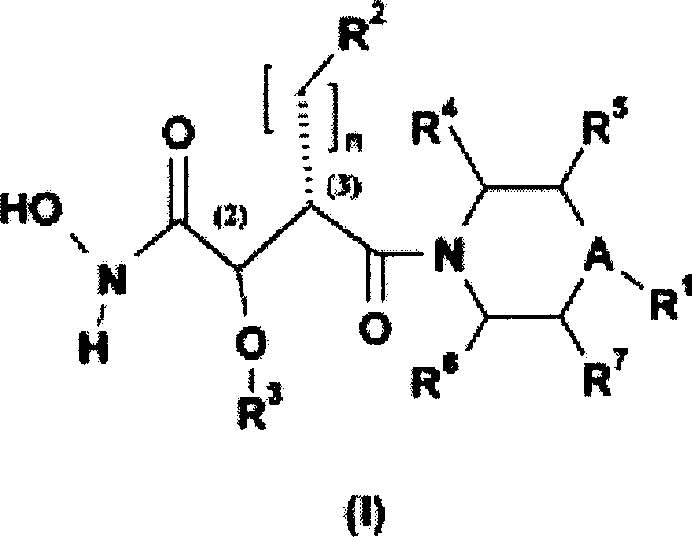
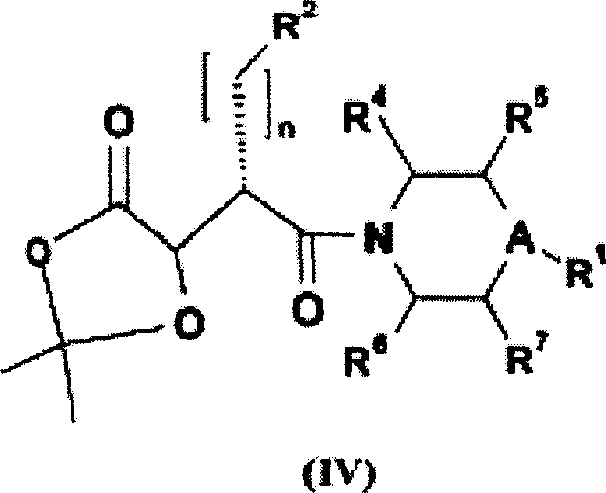
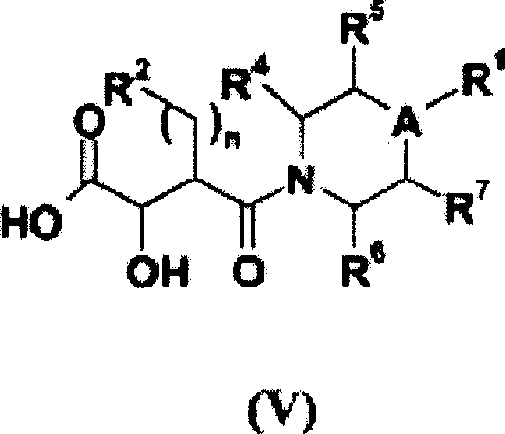
N-hydroxyamide derivatives and use thereof
A kind of hydroxy amide, derivative technology, applied in N-hydroxy amide derivative and its application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0842] Example 1: (2S,3S)-N,2-dihydroxy-5-methyl-3-{[4-(2-pyridyl)-1-piperazinyl]carbonyl}hexanamide (1)
[0843]
[0844] Step a) Formation of (5S)-2,2-dimethyl-5-((1S)-3-methyl-1-{[4-(2-pyridyl)-1-piperazinyl]carbonyl}butyl base)-1,3-dioxolan-4-one
[0845]
[0846] To (2S)-2-[(4S)-2,2-dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester and ( 2R)-2-[(4S)-2,2-Dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester (intermediate 1 ; 792.6mg; 2.0mmol; 1.0eq) of the 55 / 45 diastereoisomer mixture in DMF (15mL) was added 1-(2-pyridyl)piperazine (326.5mg; 2.0mmol; 1.0eq ). After 14 hours at room temperature, the solvent was evaporated to an oil. Chromatography (SiO 2 ) to afford the title compound as a colorless oil (50 / 50 mixture of 2 diastereoisomers). This product was dissolved in iPrOH (10 mL) and kept at -20°C for 48 hours. The supernatant was collected and evaporated to a colorless oil (301 mg, 40%, single ...
Embodiment 2
[0849] Example 2: (2S,3S)-N,2-dihydroxy-5-methyl-3-{[4-(3-phenyl-1,2,4-thiadiazol-5-yl)-1 -piperazinyl]carbonyl}hexanamide (2)
[0850]
[0851] Step a) Formation of (5S)-2,2-dimethyl-5-((1S)-3-methyl-1-{[4-(3-phenyl-1,2,4-thiadiazole- 5-yl)-1-piperazinyl]carbonyl}butyl)-1,3-dioxolan-4-one
[0852]
[0853] To (2S)-2-[(4S)-2,2-dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester and ( 2R)-2-[(4S)-2,2-Dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester (intermediate 1 ; 150.0 mg; 0.38 mmol; 1.0 equiv) to a solution of the 55 / 45 diastereoisomer mixture in DMF (1.5 mL) was added 3-phenyl-5-piperazinyl-1,2,4-thia Oxadiazole (93.2 mg; 0.38 mmol; 1.0 eq) and the resulting reaction mixture was stirred at room temperature for 14 hours. Aqueous HCl (1 N) was added, the resulting mixture was extracted with EtOAc, and dissolved in MgSO 4 Dry, filter and evaporate to an oil. Residue with CD 3 OD extraction. The prec...
Embodiment 3
[0856] Example 3: (2S,3S)-N,2-dihydroxy-5-methyl-3-({(2R)-2-methyl-4-[4-(trifluoromethyl)pyridine-2- Base]piperazin-1-yl}carbonyl)caproamide (3)
[0857]
[0858] Step a) Formation of (5S)-2,2-dimethyl-5-[(1S)-3-methyl-1-({(2R)-2-methyl-4-[4-(trifluoroform Base) pyridin-2-yl] piperazin-1-yl} carbonyl) butyl] -1,3-dioxolan-4-one
[0859]
[0860] To (2S)-2-[(4S)-2,2-dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester and ( 2R)-2-[(4S)-2,2-Dimethyl-5-oxo-1,3-dioxolan-4-yl]-4-methylpentanoic acid pentafluorophenyl ester (intermediate 1 ; 484.8mg; 1.22mmol; 1.0eq) of the 55 / 45 diastereomer mixture and triethylamine (339.13μl; 2.45mmol; 2.0eq) in DMF (10.0mL) was added (3R)-3- Methyl-1-[4-(trifluoromethyl)pyridin-2-yl]piperazine (Intermediate 9; 300 mg; 1.22 mmol; 1.0 equiv). After 14 hours of reaction at room temperature, the solvent was evaporated and the residue was dissolved in ether and extracted with water (3x). The combined organic l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com