Method of diagnosing myasthenia gravis and kits therefor
一种重症肌无力、试剂盒的技术,应用在免疫学领域,能够解决不能被用来预测患者严重性、血清抗体浓度差异极大等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
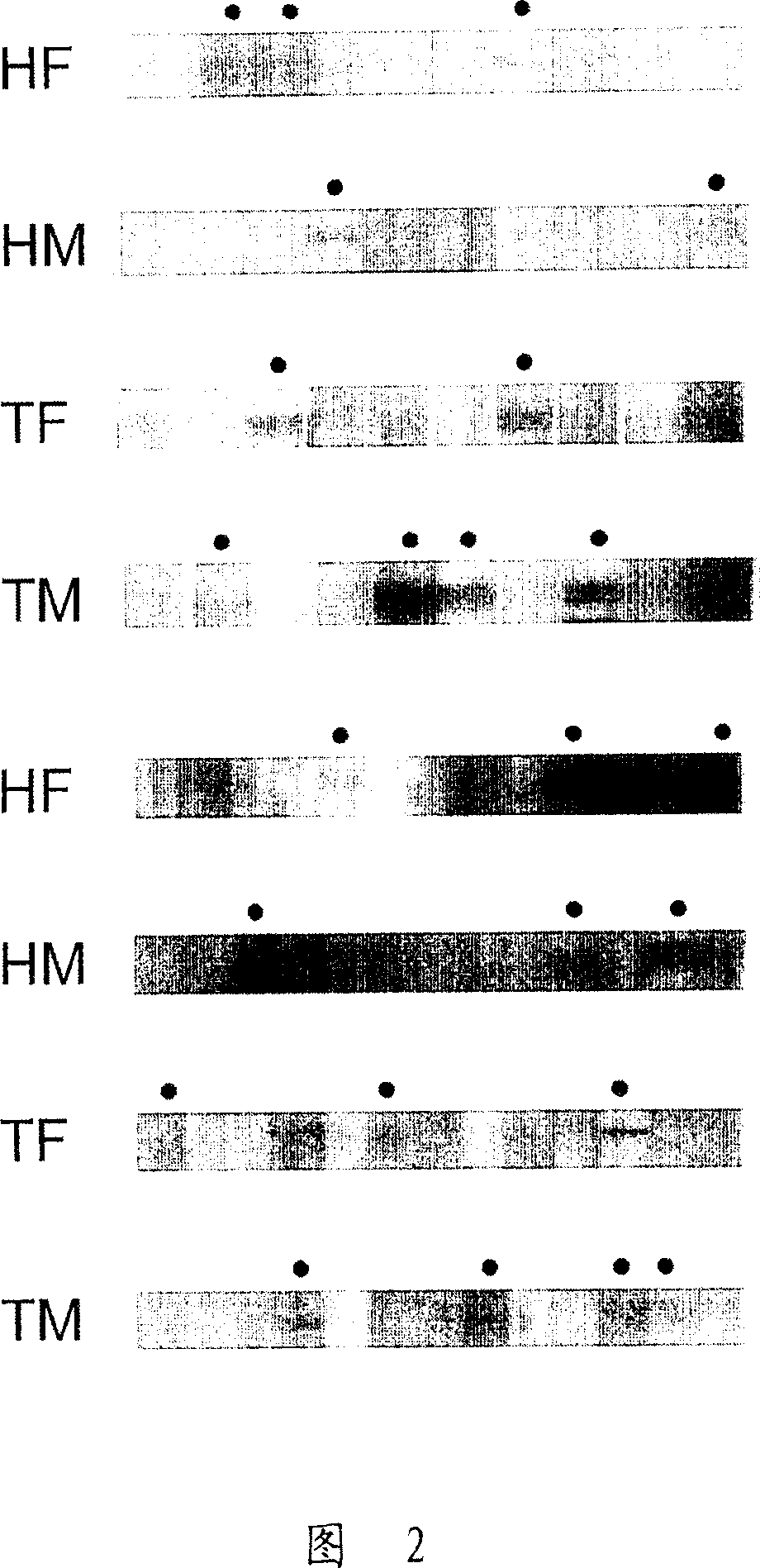
[0080] Example 1: Identification of Autoantibodies by Western Blot
[0081] Serum samples were obtained from 209 patients with myasthenia gravis at Shin Kong Hospital in Taiwan, Republic of China. These patients can be divided into four categories according to their thymus pathology: 37 patients had different degrees of thymic atrophy; 121 patients had thymoma; 40 patients had a certain degree of thymic hyperplasia (thymic hyperplasia); and 11 patients had unknown thymic pathology. In addition, 76 patients had type I MG, 81 had type IIa MG, 38 had type IIb MG, and 14 had type III or IV MG. Serum samples from the negative control group were 54 patients from Taichung Veterans General Hospital who suffered from another unrelated, non-autoimmune disease, Membranous glomerulonephritis (MGN). ).
[0082] Cell lysates of HepG2 / C3A cells were separated by 10% SDS-PAGE gel, loaded with 20 μg of cell extract per lane and 2 μg of hsp60 or hsp90 protein per lane. During electrophoresi...
Embodiment 2
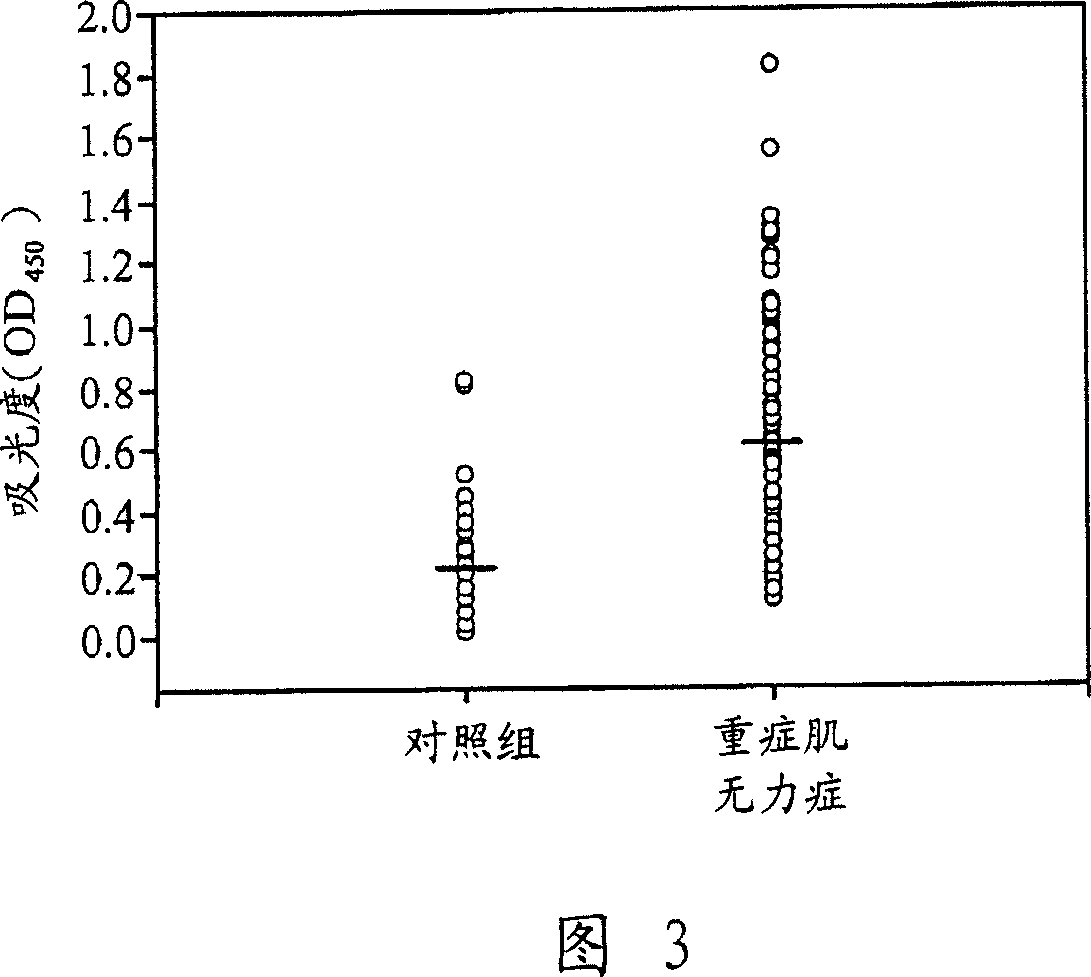
[0085] Example 2: Measurement of Autoantibodies by Enzyme-Linked Immunosorbent Assay (ELISA)
[0086] Aliquots of serum samples from Example 1 were further analyzed by ELISA. ELISA microplate (Coming Life Sciences, New York, NY, USA) was dissolved in 0.1M NaHCO 3 0.2 μg of human hsp60 (purified from E. coli) or hsp90 (purified from S. cerevisiae) in pH=8.6 was coated overnight at 4°C. The above two recombinant heat shock proteins were purchased from Sigama-Aldrich Co. The coated microwell plate was washed 3 times with PBS, each time for 1 minute, and then incubated with 200 μl of blocking solution (5 mg / ml BSA in PBST) at 37°C for 1 hour, and each well was washed with PBST for 6 hours. times, 1 minute each time. The plates were then reacted with patient serum diluted 1:100 to 1:800 in blocking solution at 37°C for 1 hour and 30 minutes. After incubation with the primary antibody, wash the microwell plate 6 times with blocking solution for 1 minute each time, and then incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com