Optimized recombinant antigen polypeptide Abeta1-15-HSP60 nucleotide sequence and high-efficiency preparation method thereof
A technology of nucleotide sequence and recombinant antigen, which is applied in the field of high-efficiency preparation, can solve the problem of low expression of recombinant protein, and achieve the effects of high solubility, low cost and simple purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Recombinant Antigen Polypeptide Aβ 1-15 - Optimization of HSP60 nucleotide sequence
[0046] Recombinant antigenic polypeptide Aβ of the present invention 1-15 -The whole gene synthesis of HSP60 is completed by GenScript Biotechnology Co., Ltd. When performing gene synthesis, two enzyme cutting sites, Nde I and Xba I, respectively designed at the upper and lower ends of the target gene, are required to be CGCCATATG and ACGCTCTAGA. The synthesized target gene is 96 bp in full length, loaded into the cloning vector pUC57 plasmid and verified by sequencing. native antigen polypeptide Aβ 1-15 -Compared with the HSP60 gene, the cDNA synthesized by the present invention changes part of the codons into Escherichia coli preferred codons, and at the same time mutates the ACA codons, but does not involve amino acid changes, which is a synonymous mutation.
Embodiment 2
[0048] Recombinant expression vector pCold / Aβ 1-15 - Construction of HSP60
[0049] (1) will carry full-length Aβ 1-15 - The pUC57 and pCold plasmids of HSP60 were respectively digested with Nde I and Xba I, and the two fragments were ligated with T4 ligase to obtain a ligation product.
[0050] (2) Transform the ligation product into Escherichia coli DH5α competent cells by heat stimulation, select clones on the LB plate containing ampicillin, prepare a small amount of plasmids, and screen positive clones by double enzyme digestion and sequencing to obtain pCold / Aβ 1-15 - HSP60 plasmid.
Embodiment 3
[0052] Aβ 1-15 -Construction of HSP60 High Efficiency Soluble Expression Engineering Bacteria
[0053] (1) The recombinant plasmid obtained in Example 2 was transformed into the host bacterium BL21(DE3), which is the engineering bacterium of the present invention.
[0054] (2) Select a single clone on a solid M9 agar medium plate, shake the bacteria overnight in 4mL M9 liquid medium containing 100ug / ml ampicillin at 37°C, 200 rpm.
[0055] (3) Inoculate 1% of the bacterial solution into the M9 culture medium containing 100ug / ml ampicillin, shake the bacteria at 37°C to OD=0.5, add 1mM IPTG at a final concentration of induction, shake the bacteria at 15°C for 6h, and recover the bacteria.
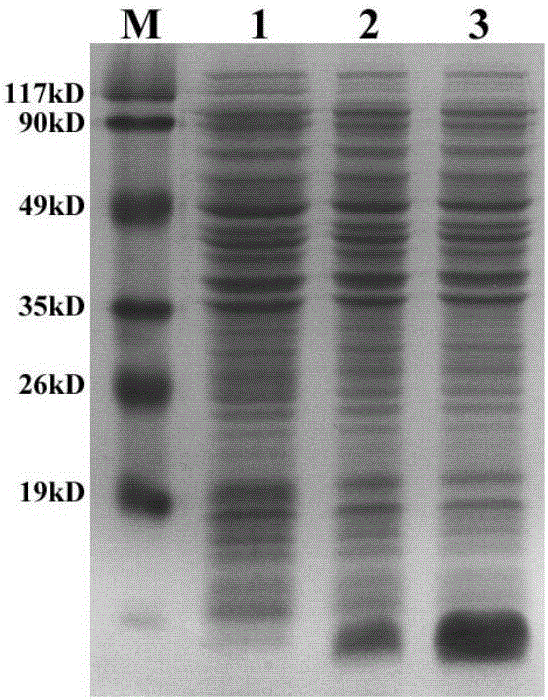
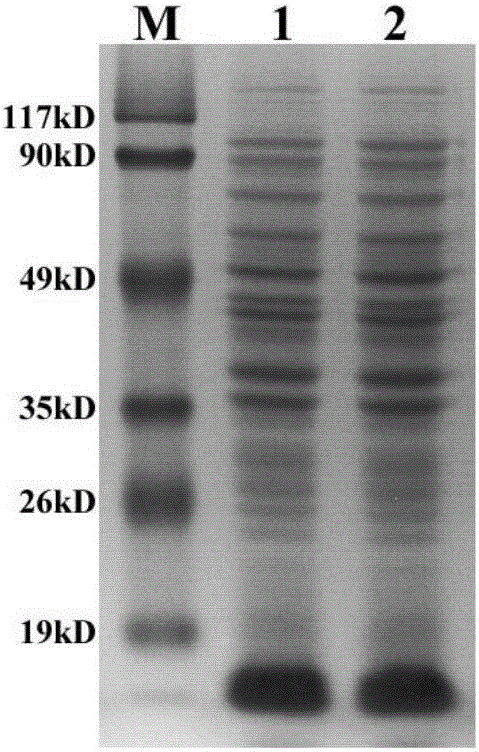
[0056] (4) After the bacteria were disrupted by ultrasound, SDS-PAGE was performed on the whole bacteria, supernatant and precipitated (inclusion body) proteins to analyze the expression of exogenous proteins. Control the changes of exogenous proteins in the bacteria and supernatant before...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com