Methods, systems, and kits for intravascular nucleic acid delivery
a nucleic acid and intravascular technology, applied in the field of medical methods and devices, can solve the problems of affecting the viability of vascular smooth muscle cells, and requiring high temperatures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
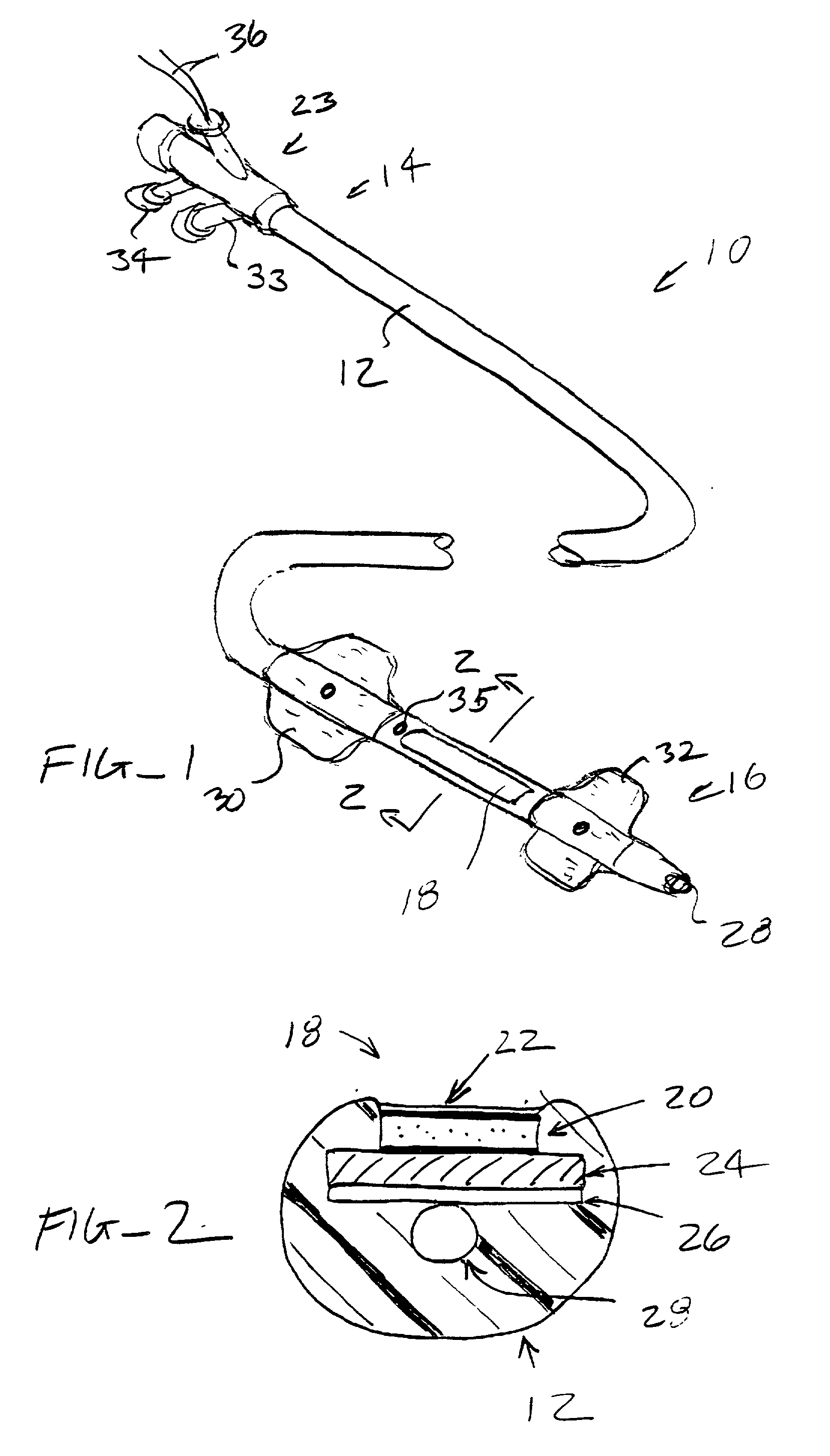
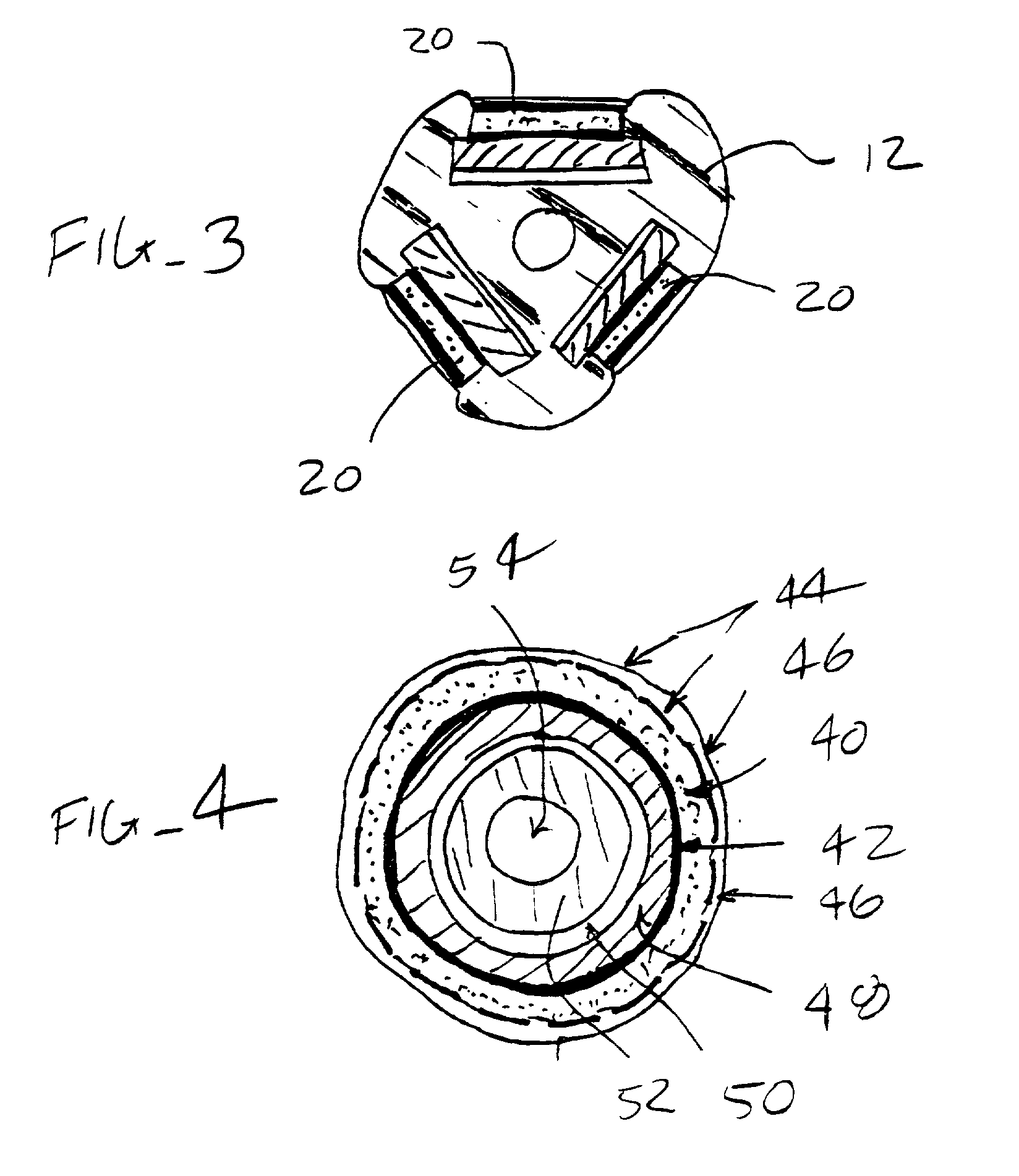
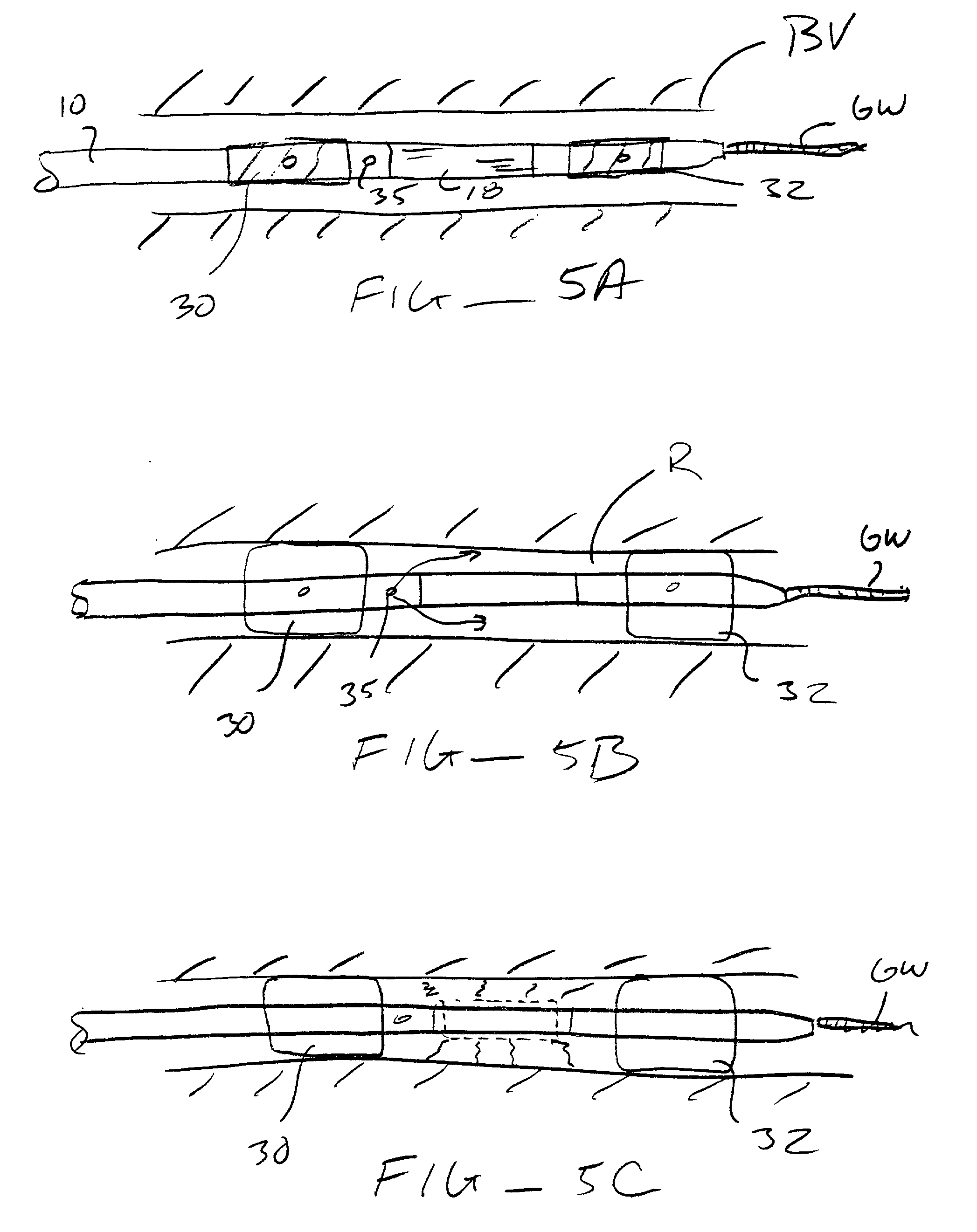
[0035] The nucleic acids delivered by the methods and devices of the present invention will comprise nucleic acid molecules in a form suitable for uptake into target cells within a host tissue, usually smooth muscle cells lining the blood vessels. The nucleic acids will usually be in the form of bare DNA or RNA molecules, where the molecules may comprise one or more structural genes, one or more regulatory genes, antisense strands, strands capable of triplex formation, or the like. Commonly, such nucleic acid constructs will include at least one structural gene under the transcriptional and translational control of a suitable regulatory region. Optionally, but not necessarily, the nucleic acids may be incorporated in a viral, plasmid, or liposome vesicle delivery vehicle to improve transfection efficiency.
[0036] If viral delivery vehicles are employed, they may comprise viral vectors, such as retroviruses, adenoviruses, and adeno-associated viruses, which have been inactivated to pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com