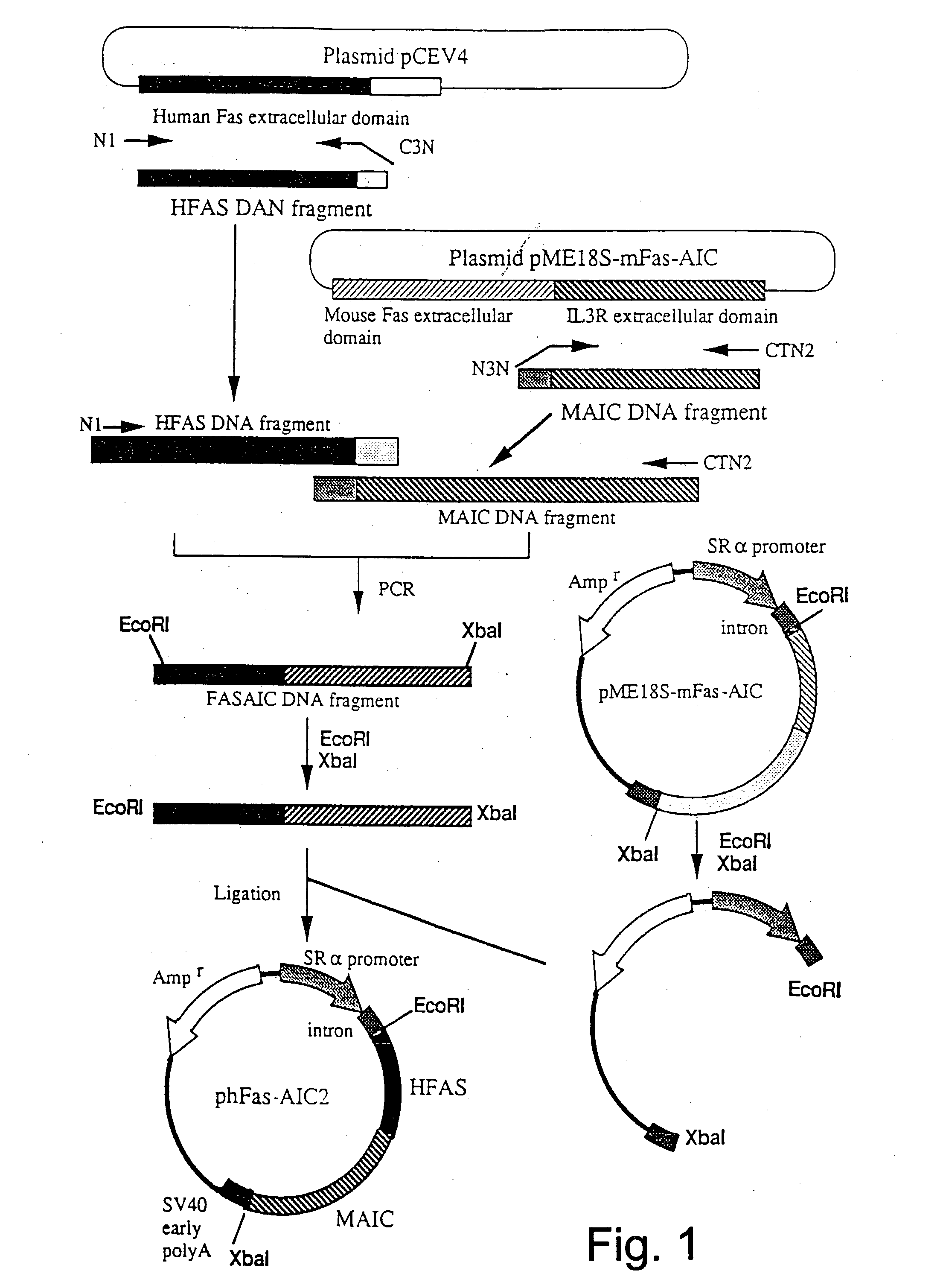
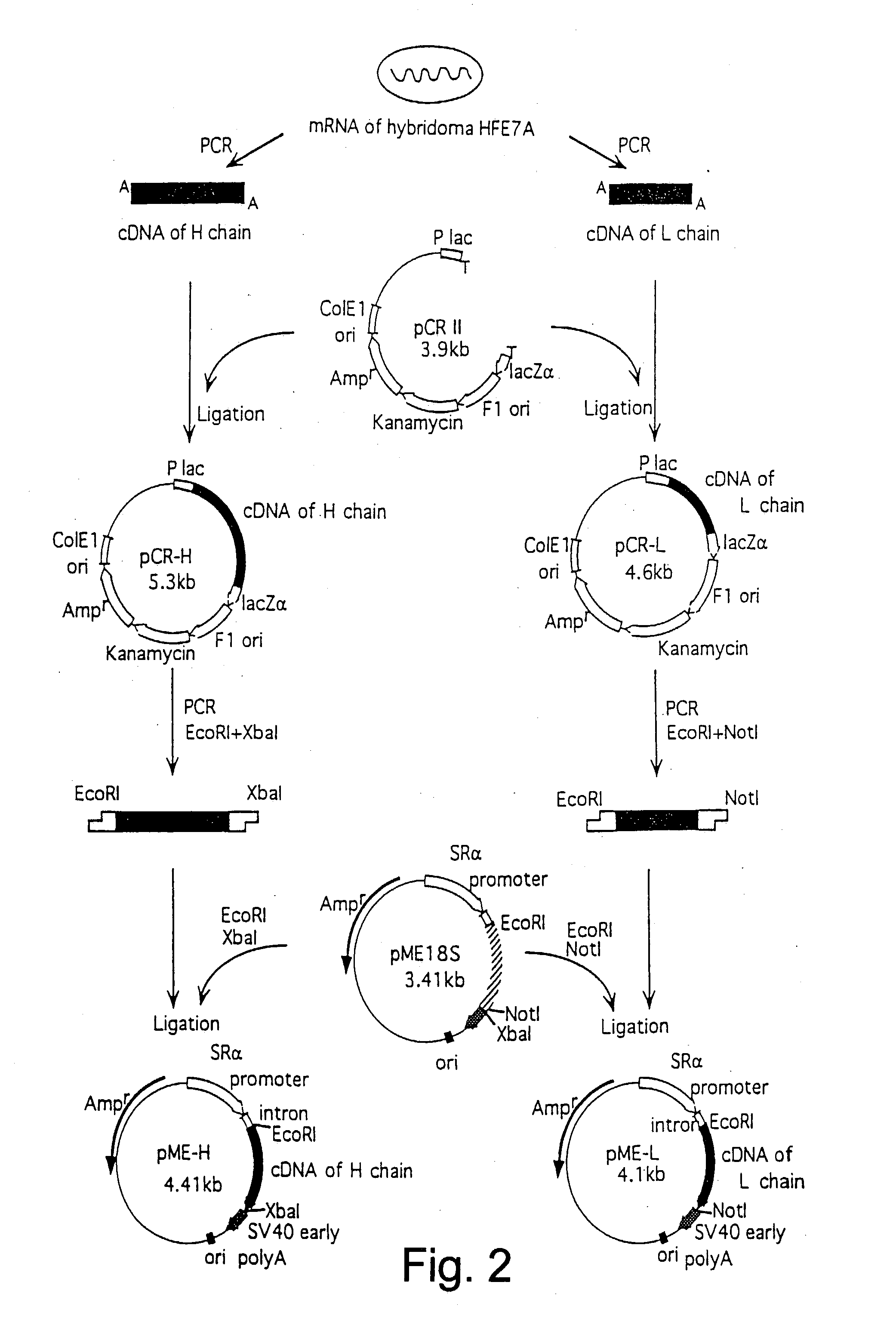
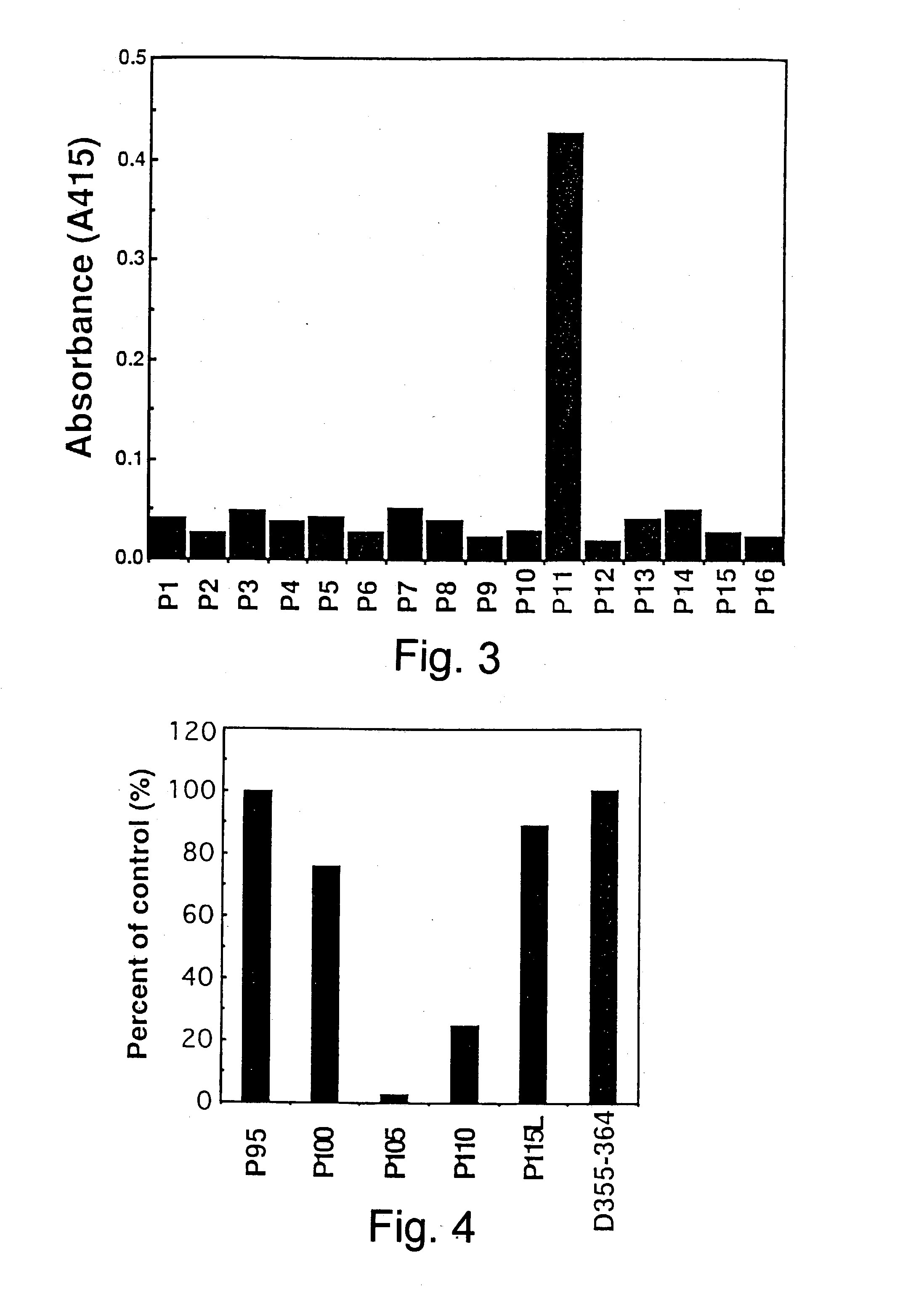
Anti-Fas antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 2
Immunization of Mice and Preparation of Hybridoma
[0420] (2-1) Immunization
[0421] A sample of 1 ml of the crude human Fas fusion protein solution obtained in Reference Example 1 above (total protein: 100 .mu.g) was taken and, to this, were added 25 .mu.l of 2N HCl , 250 .mu.l of 9% w / v potash alum (final concentration: 1.1% w / v) and 25 .mu.l of 2N NaOH. The resulting mixture was adjusted to a pH of between about 6.5 and 7.0 by the addition of about 120 .mu.l of an aqueous solution of 10% (w / v) sodium hydrogencarbonate and left to stand at room temperature for about 30 minutes. After this time, 200 .mu.l of killed Bordetella pertussis (Wako Pure Chemical Industries, Ltd.; 1.2.times.10.sup.11 cells / ml) were added to the mixture in order to activate the T cells, and the mixture was administered intraperitoneally to a Fas knock-out mouse. The mouse used was prepared in accordance with the method described by Senju et al. [c.f. Senju, S. et al., (1996), International Immunology, 8, 423]. ...
reference example 3
Purification of HFE7A Monoclonal Antibody
[0437] The mouse-mouse hybridoma HFE7A obtained in Reference Example 2 (FERM BP-5828) was grown to a cell density of 1.times.10.sup.6 cells / ml by incubation in 1 l of ASF medium, containing 10% v / v FCS, at 37.degree. C. under 5% v / v CO.sub.2. The culture was then centrifuged (1,000 r.p.m., 2 minutes) and the supernatant discarded. The cell pellet was washed once with serum-free ASF medium, suspended in 1 l of serum-free ASF medium and incubated for 48 hours at 37.degree. C. under 5% v / v CO.sub.2. After this time, the culture was centrifuged (1,000 r.p.m. for 2 minutes) to recover the supernatant. This supernatant was then placed in a dialysis tube (exclusion m.w.: 12,000-14,000; Gibco BRL), and dialyzed against 10 volumes of 10 mM sodium phosphate buffer (pH 8.0). Partial purification of IgG from the inner solution was achieved using a high performance liquid chromatography apparatus (FPLC system; Pharmacia) under the following conditions:
[04...
reference example 4
[0445] (4-1) Preparation of Poly(A).sup.+ RNA
[0446] Cells of the mouse-mouse hybridoma HFE7A (FERM BP-5828), obtained in Reference Example 2, were grown to a cell density of 1.times.10.sup.6 cells / ml in 1 l of ASF medium supplemented with 10% v / v FCS at 37.degree. C. under 5% v / v CO.sub.2. These cells were harvested by centrifugation and lyzed in the presence of guanidinium thiocyanate solution [4 M guanidinium thiocyanate, 1% v / v Sarcosyl, 20 mM EDTA, 25 mM sodium citrate (pH 7.0), 100 mM 2-mercaptoethanol, 0.1% v / v Antifoam A] and the lysate was recovered. Isolation of poly(A).sup.+ RNA was performed essentially as described in "Molecular Cloning A Laboratory Manual" [c.f. Maniatis, T., et al., (1982), pp. 196-198]. More specifically, the procedure was as follows.
[0447] The recovered cell lysate was sucked into and exhausted from a 10 ml-syringe equipped with a 21-gauge needle, several times. The cell lysate was layered over 3 ml of an aqueous solution of 5.7 M cesium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com