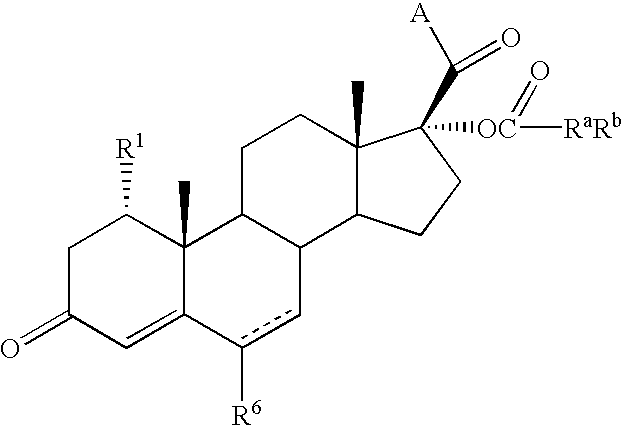
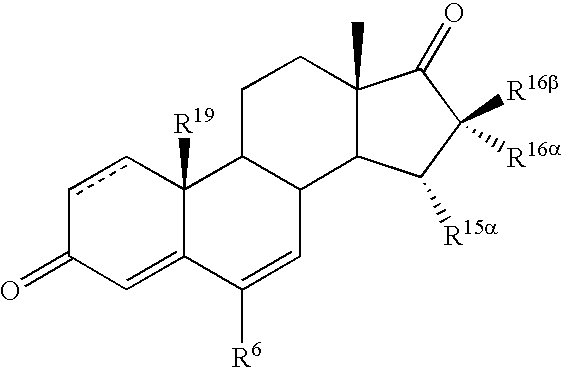
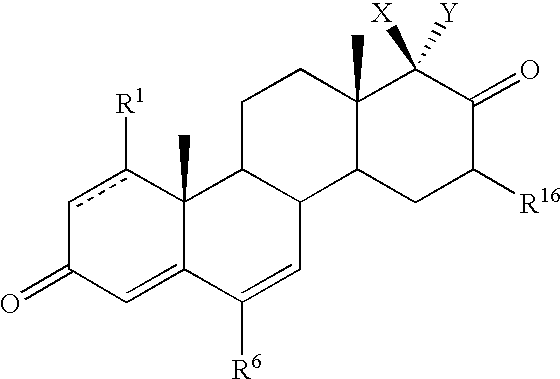
Inhibitors of type 5 and type 3 17beta-hydroxysteroid dehydrogenase and methods for their use
a technology of 17beta-hydroxysteroid and dehydrogenase, which is applied in the field of androgen-sensitive inhibitors, can solve the problems of benign prostatic hyperplasia responding unfavorably, unfavorable benign prostatic hyperplasia, and undesirable initial upward spike of androgenic production and secretion by the testes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 6-methyl 4,6-pregnadien / 1,4,6-pregnatrien-17-ol-3,20-dione Alkanoates / Benzoates
[0556] These syntheses are described in Schemes 1 and 2. 71 72
example 1a
[0557] 6-methyl-1,4,6-Pregnatrien-17.alpha.-ol-3,20-dione acetate (2; EM-910):
[0558] Megestrol acetate (2.5 g; 6.7 mmol) and DDQ (5.07 g; 22 mmol) in dioxane (30 mL) were refluxed for 2 h. Solvent was removed and the mixture in EtOAc was washed with saturated NaHCO.sub.3 solution (3 times). Solvent was dried (MgSO.sub.4) and evaporated to give the product. Purification on silica gel column (hexanes / acetone) gave the trienone (2.1 g) in 90% yield; IR (KBr, cm.sup.-1) 2953, 2895, 1729, 1709, 1658, 1646, 1610, 1365, 1264, 1247, 887. .sup.1H NMR (CDCl.sub.3) .delta. 0.69 (s, 3H, H-C18), 1.13 (s, 3H, H-C19), 1.87 (s, 3H, 6-CH3), 2.0 (s, 3H) 2.02 (s, 3H), 2.89-2.98 (m, 1H), 5.79 (s, 1H), 6.22 (s, 1H), 6.20-6.24 (m, 1H, 2-H), 7.04 (d, 1H, 1-H, J=10 Hz); .sup.13C NMR (CDCl.sub.3) .delta. 203.7, 186.4, 170.6, 163.2, 153.4, 134.5, 131.8, 127.7, 121.6, 96.2, 49.1, 47.9, 47.1, 41.1, 37.7, 31.1 30.3, 26.4, 23.2, 21.5, 20.5 19.2, 14.4.
example 1b
[0559] 6-methyl-4,6-Pregnadien-17.alpha.-ol-3,20-dione (3): Megestrol acetate (11.2 g) was dissolved in 200 mL of boiling MeOH and to this, was added 2N NaOH (20 mL). The mixture was refluxed for 5 h. Approx. 100 mL of MeOH was removed, and water (50 mL) was added and the suspension was cooled to 0.degree. C. to give a solid, which was filtered and washed with water (9.6 g, 96%); IR (KBr, cm.sup.-1) 3493, 2942, 2767, 1702, 1658, 1646, 1623, 1575. .sup.1H NMR (CDCl.sub.3) .delta. 0.75 (s, 3H, H-C18), 1.06 (s, 3H, H-C19), 1.81 (s, 3H, 6-CH.sub.3), 2.25 (s, 3H), 2.96 (s, 1H), 5.83 (s, 1H), 5.95 (s, 1H); .sup.13C NMR (CDCl.sub.3) .delta. 211.3, 200.0, 164.4, 138.5, 131.1, 121.0, 89.5, 50.3, 48.7, 47.9, 36.9, 36.1, 34.0 33.5, 30.2, 27.7, 23.3, 20.1, 19.8 16.3, 15.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com