Neisseria meningitidis polypeptide, nucleic acid sequence and uses thereof
a technology of neisseria meningitidis and polypeptide, which is applied in the field of polypeptides, can solve the problems of fatal meningitis, limited immune response in infants, and limited immune response, and achieves broad cross-strain protection and enhances pathogen clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
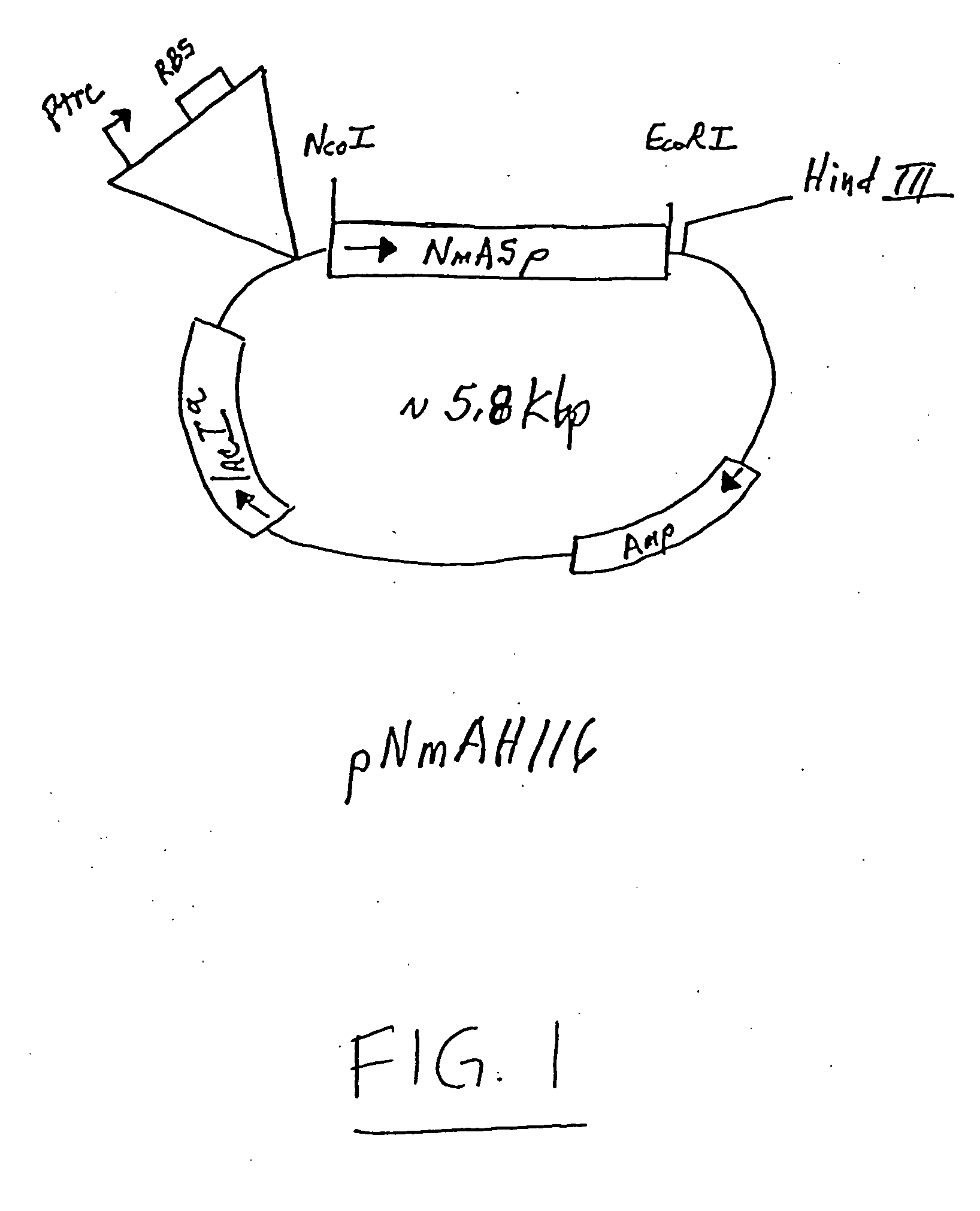
5.1. NMASP Polypeptide
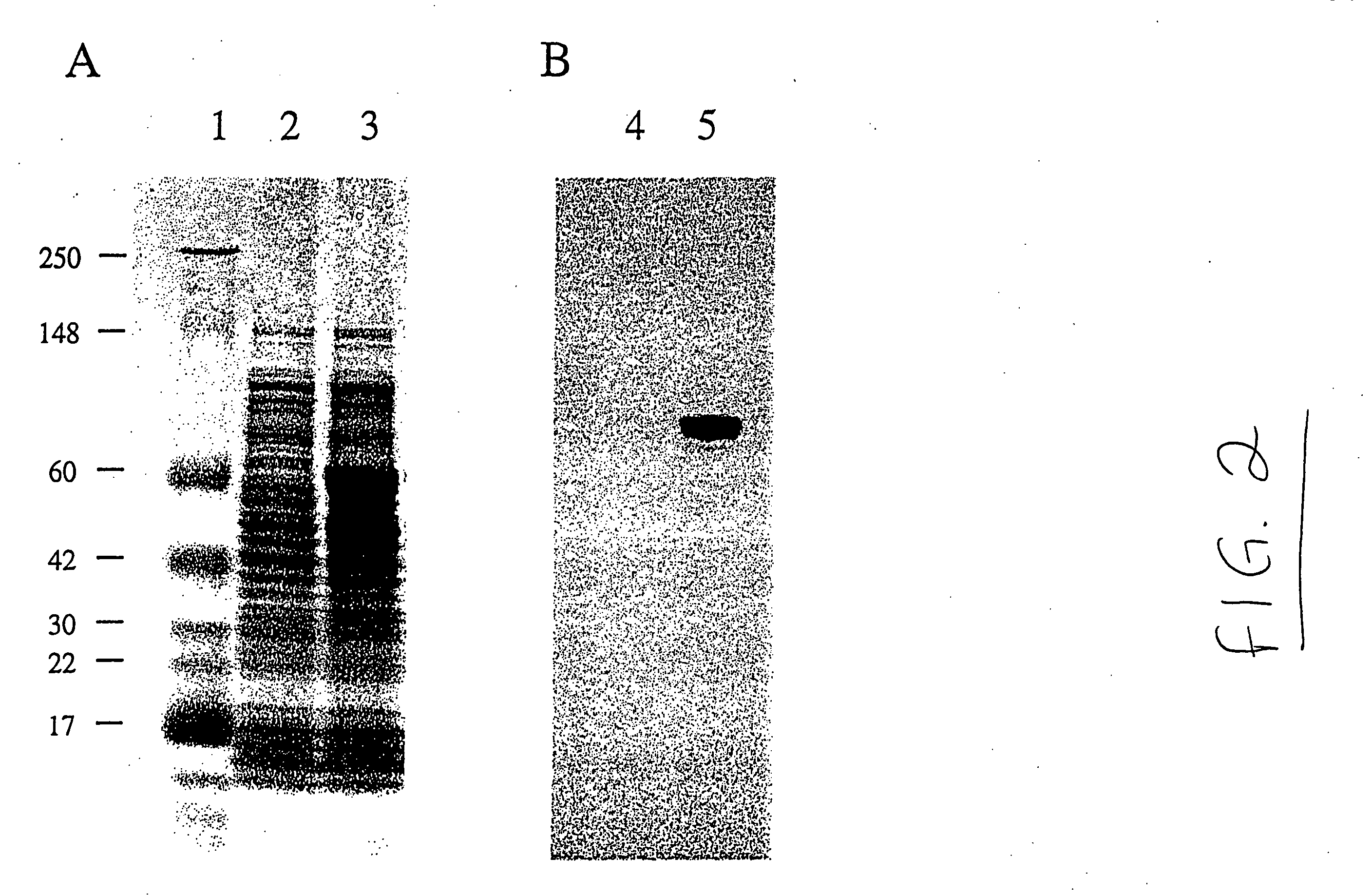
[0042] The invention provides an isolated or a substantially pure native (wild type) or recombinantly produced polypeptide, referred to as NMASP, of Neisseria meningitidis, and various strains or cultivars thereof. The NMASP polypeptide comprises the whole or a subunit of a non-cytosolic protein embedded in, or located in the bacterial envelope, which may include the inner membrane, outer surface, and periplasmic space. The NMASP polypeptide has an apparent molecular weight, as determined from the deduced amino acid sequence, of about 40 kD to about 55 kD, preferably about 44 kD to about 53 kD.
[0043] NMASP polypeptide may also be identified as the polypeptide in hydrophobic (salt) or detergent extracts of Neisseria meningitidis blebs or intact cells that has an apparent molecular weight about 40 kD to about 55 kD, preferably about 44 kD to about 53 kD, as determined by denaturing gel electrophoresis in 12% PAG with SDS, using formulations as described in Harlow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com