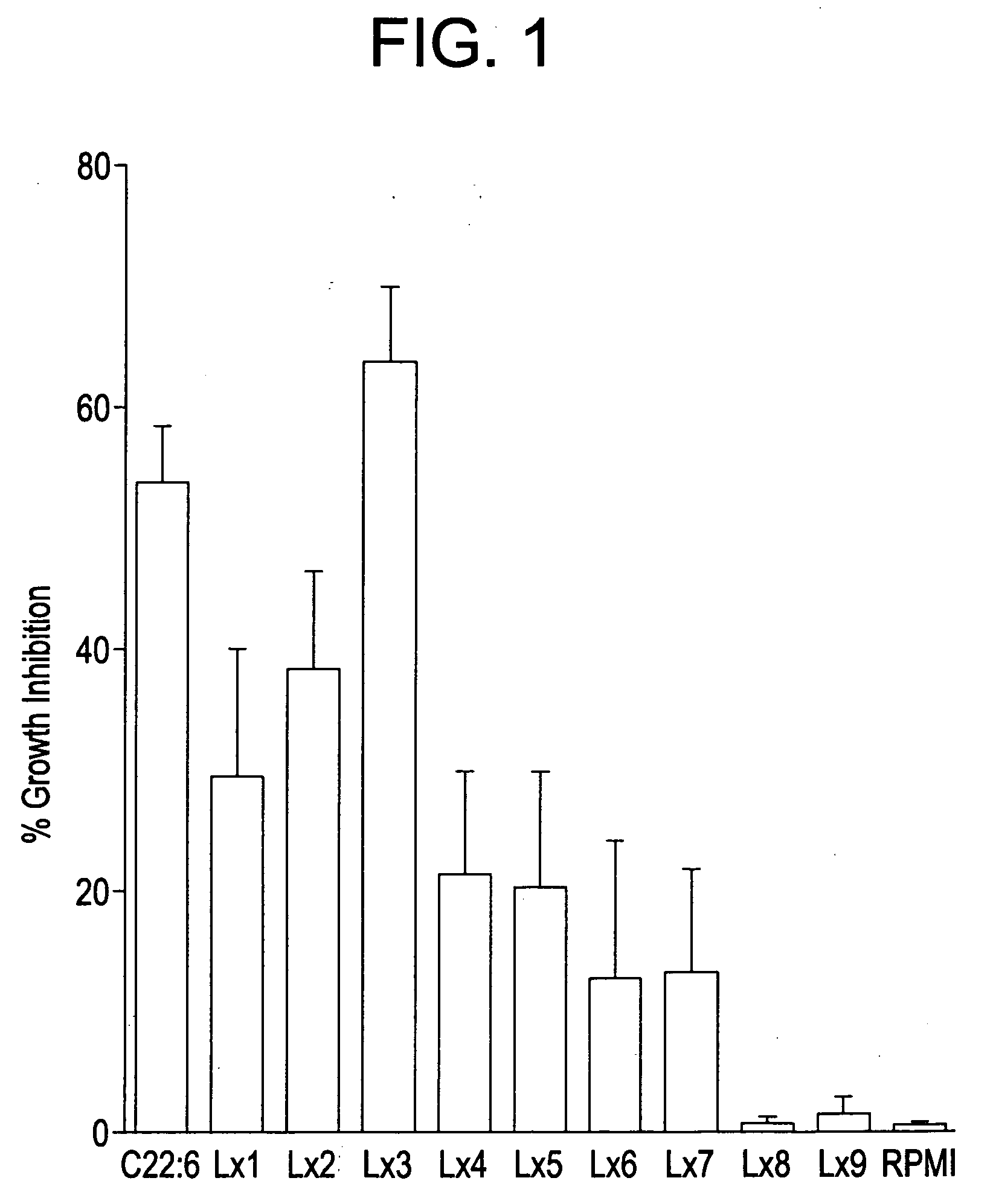
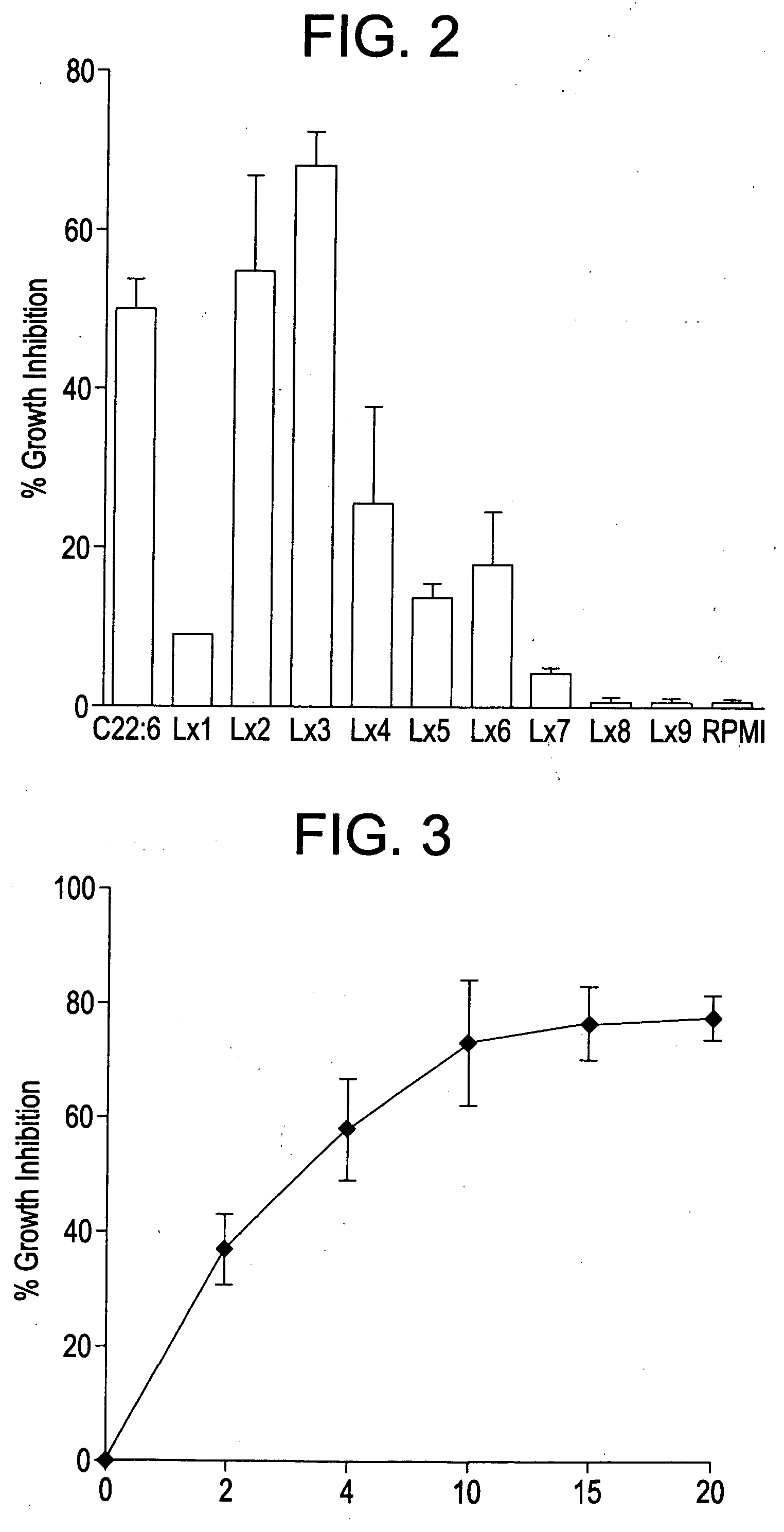
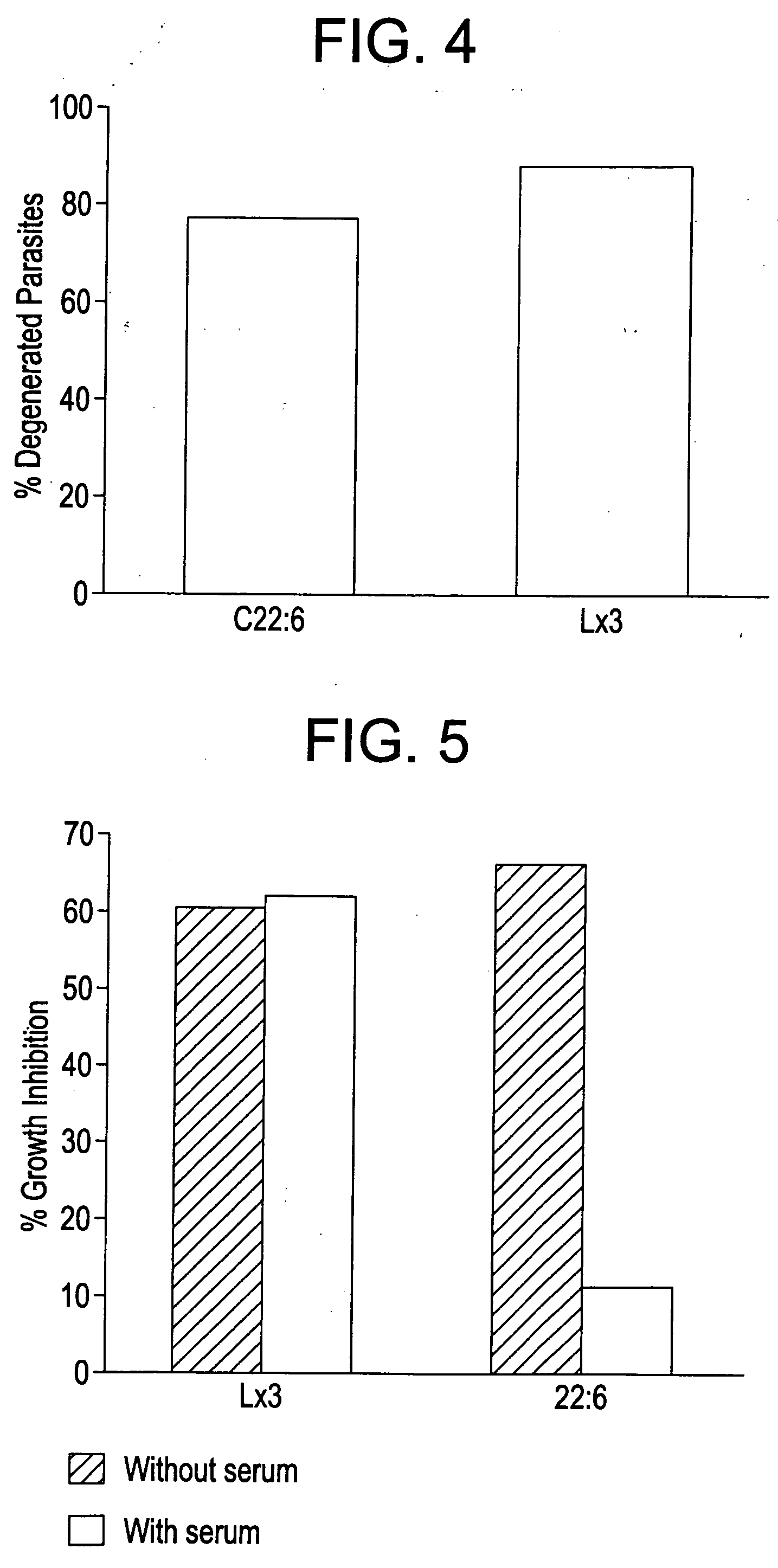
Anti-inflammatory nitro-and thia-fatty acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0054] Octadecan-1-ol (1a) was obtained from Aldrich Chemical Co. Arachidonyl alcohol (1b), linolenyl alcohol (1c), gamma linolenyl alcohol (1d) and docosahexaenyl alcohol 1e were purchased from Nu-Chek Prep. Inc. (Elysian, Minn., USA).
1-Bromooctadecane (2a); Typical Procedure
[0055] Octadecan-1-ol (1a) (520 mg, 1.92 mmol) and Ph3P (550 mg, 2.10 mmol) were dissolved in CH2Cl2 (25 mL). The mixture was cooled in an ice bath and CBr4 (630 mg, 1.90 mmol) was added with stirring. The mixture was allowed to warm to r.t. and was stirred overnight, then it was concentrated under a stream of N2 and the residue was subjected to flash column chromatography on silica, eluting with hexane, to afford 1-bromooctadecane (2a) (605 mg, 96%) as a waxy solid; mp 26-28° C.
[0056] IR (KBr): v 2920 (s), 2848 (s), 1468 (s), 1378 (w), 1254 (w), 1144 (m), 720 (m), 638 (s) cm1.
[0057]1H NMR (CDCl3, 300 MHz): δ=0.87 (t, 3H, J=6.7 Hz, C18-H3), 1.25-1.32 [m, 30H, (C3-17)-H2)], 1.82-1.85 (m, 2H, C2-H2), 3.40 (t,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap