Immune regulation
a technology of immune regulation and regulation, applied in the field of tissue and organ transplantation, can solve problems such as unreachabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody-Mediated Inhibition of Proliferation of T Cells from Class II-Engineered Animals
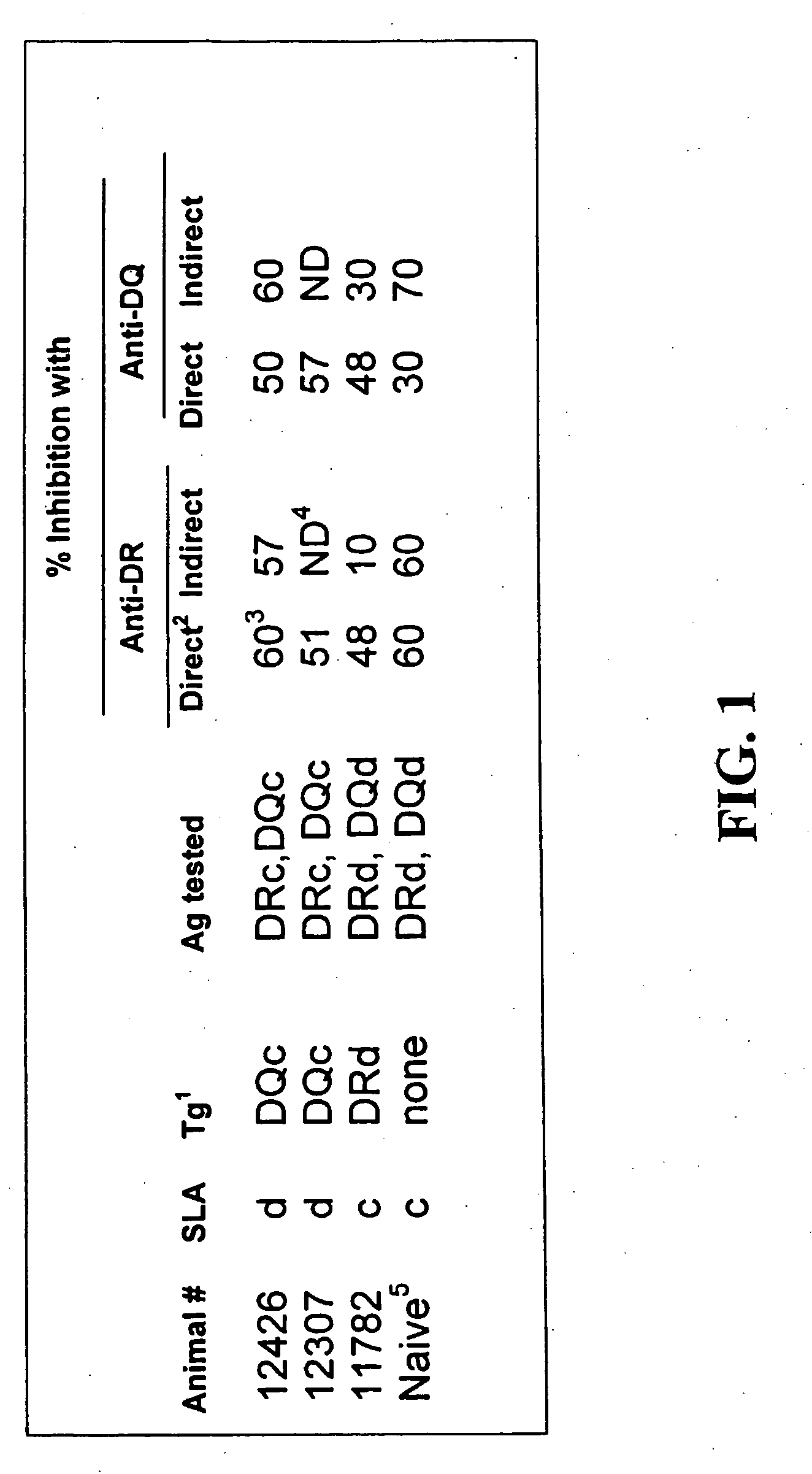
[0101] As seen in FIG. 1, the transfer of donor-class II transgenes (Tg) to an animal did not affect the ability of the recipient to respond to the transferred class II proteins through either the direct or indirect pathways.
[0102] (1). Class II transgene introduced. (2). The direct pathway of antigen presentation was assessed with purified T cells from class II engineered animals tested against donor-type irradiated PBLs. The indirect pathway was tested on recipient T cells, supplemented with self APC and stimulated by donor-type irradiated T cells. (3). The combination of anti-DR and -DQ mAbs resulted in 90-100% inhibition in all cases. (4). Not detected. (5). Results from a naïve animal representative of 5 experiments.
Example 2
Expression of Transduced MHC Class II cDNAs in Class II-Committed Cells
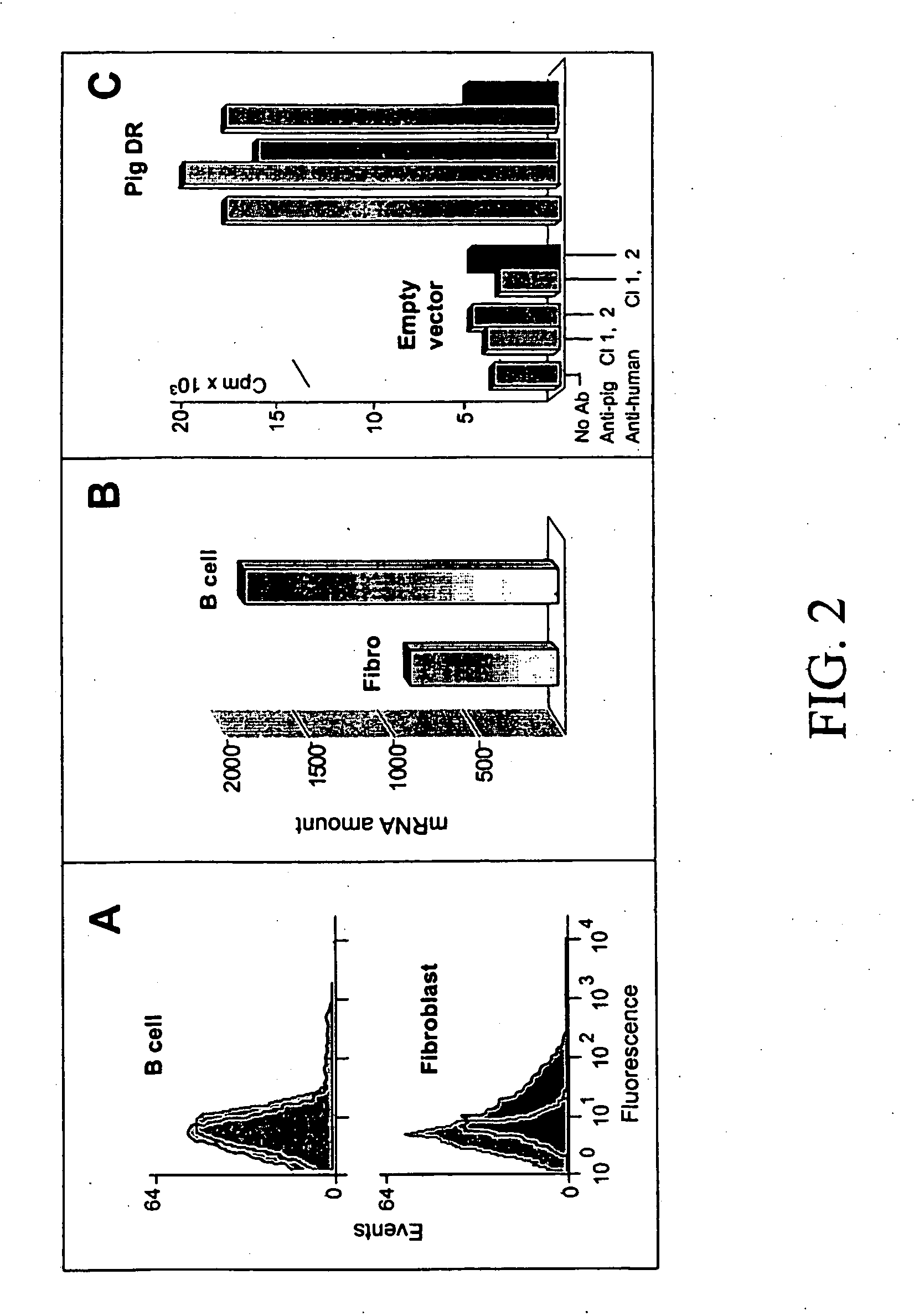
[0103] As seen in FIG. 2, flowcytometry analysis of surface protein expression from pig DRA+B...
example 3
MHC class II Peptides Docking onto Self Class II Grooves
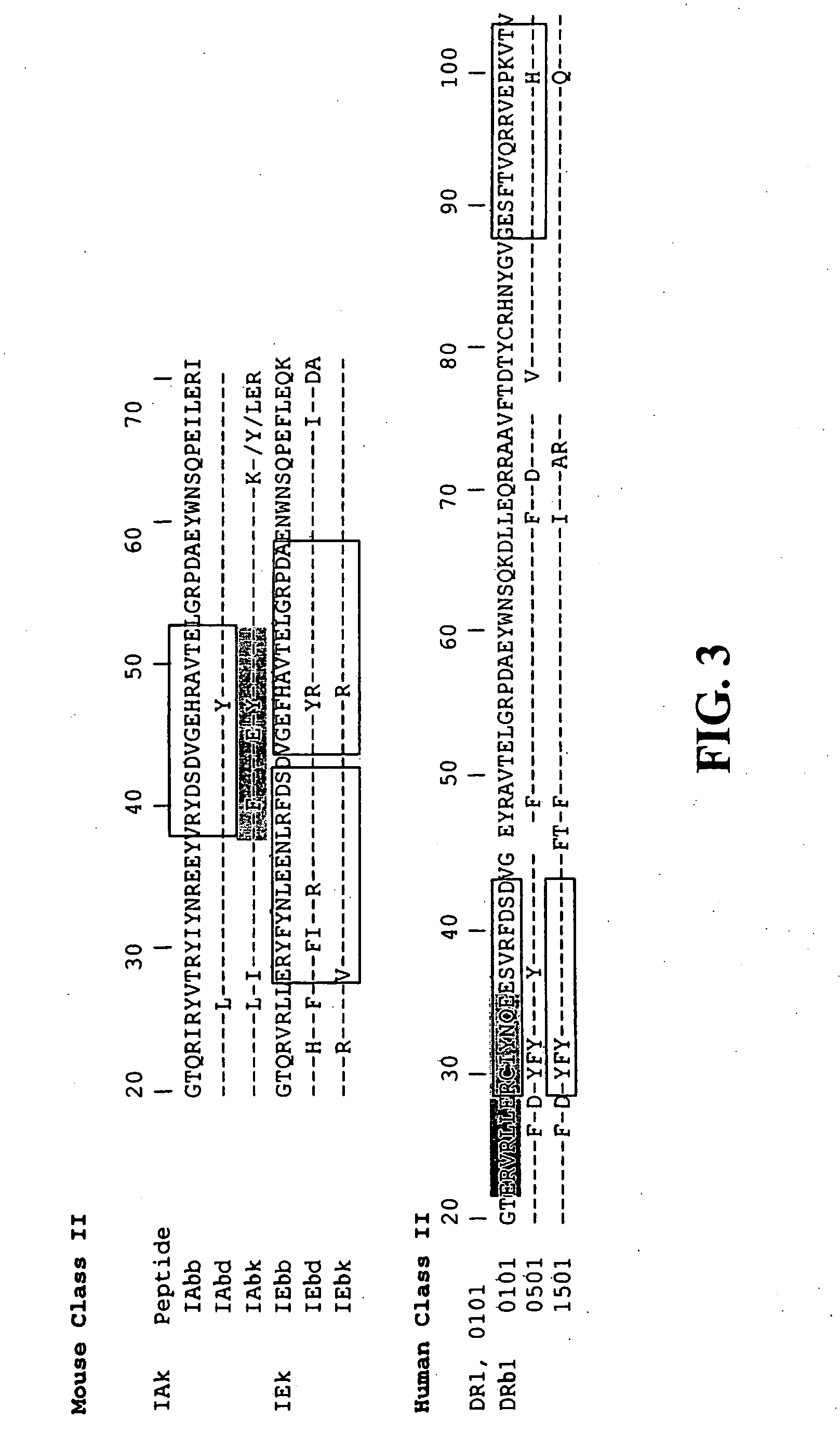
[0104] As seen in FIG. 3, computer predictions for potential high affinity class II peptide motifs docking into class II grooves identified mostly autologous Pep2Reg types, for example IA peptides binding preferentially into the IA groove. Furthermore, best fitted class II peptides preferentially derived from polymorphic regions between allelic b chains such as those from the murine IEb 27-57 and IAb 37-54 regions or human DRb1 22-44 (FIG. 3, boxes).
example 4
Flow Cytometry Analysis of CD4 Positive Subsets Stimulated by Autologous Immature DC Cells
[0105] As seen in FIG. 4, results from studies performed in the miniature swine model show that: 1) CD4+, CD25+ T-reg-like cells produced more blasts than their CD4+, CD25neg counterpart following 1 week priming in vitro with syngeneic iDC (CD1neg, CD2neg, CD3neg, Monocytepos, class IIpos), (FIG. 4, R3 gate); 2) T-reg-like cells also expressed marker of activation such as CTLA4 (FIG. 4, lower right panel).
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com