Battery including carbon foam current collectors
a carbon foam and current collector technology, applied in the direction of cell components, electrochemical generators, nickel accumulators, etc., can solve the problems of lead-acid batteries, corrosion of lead current collectors of positive plates, and constant corrosion of current collectors of each positive pla
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
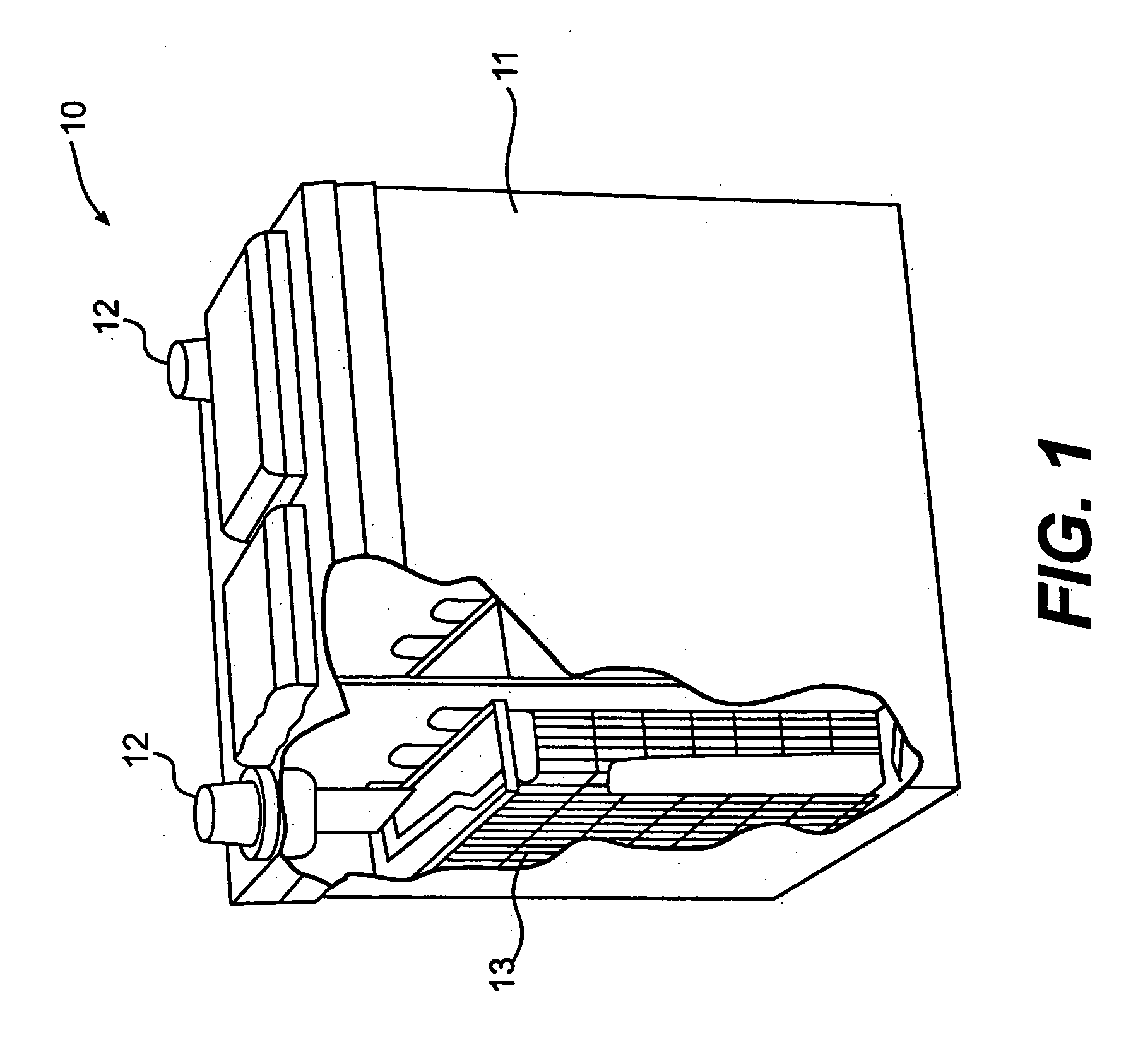
[0017]FIG. 1 illustrates a battery 10 in accordance with an exemplary embodiment of the present invention. Battery 10 includes a housing 11 and terminals 12, which are external to housing 11. At least one cell 13 is disposed within housing 11. While only one cell 13 is necessary, multiple cells may be connected in series or in parallel to provide a desired total potential of battery 10.
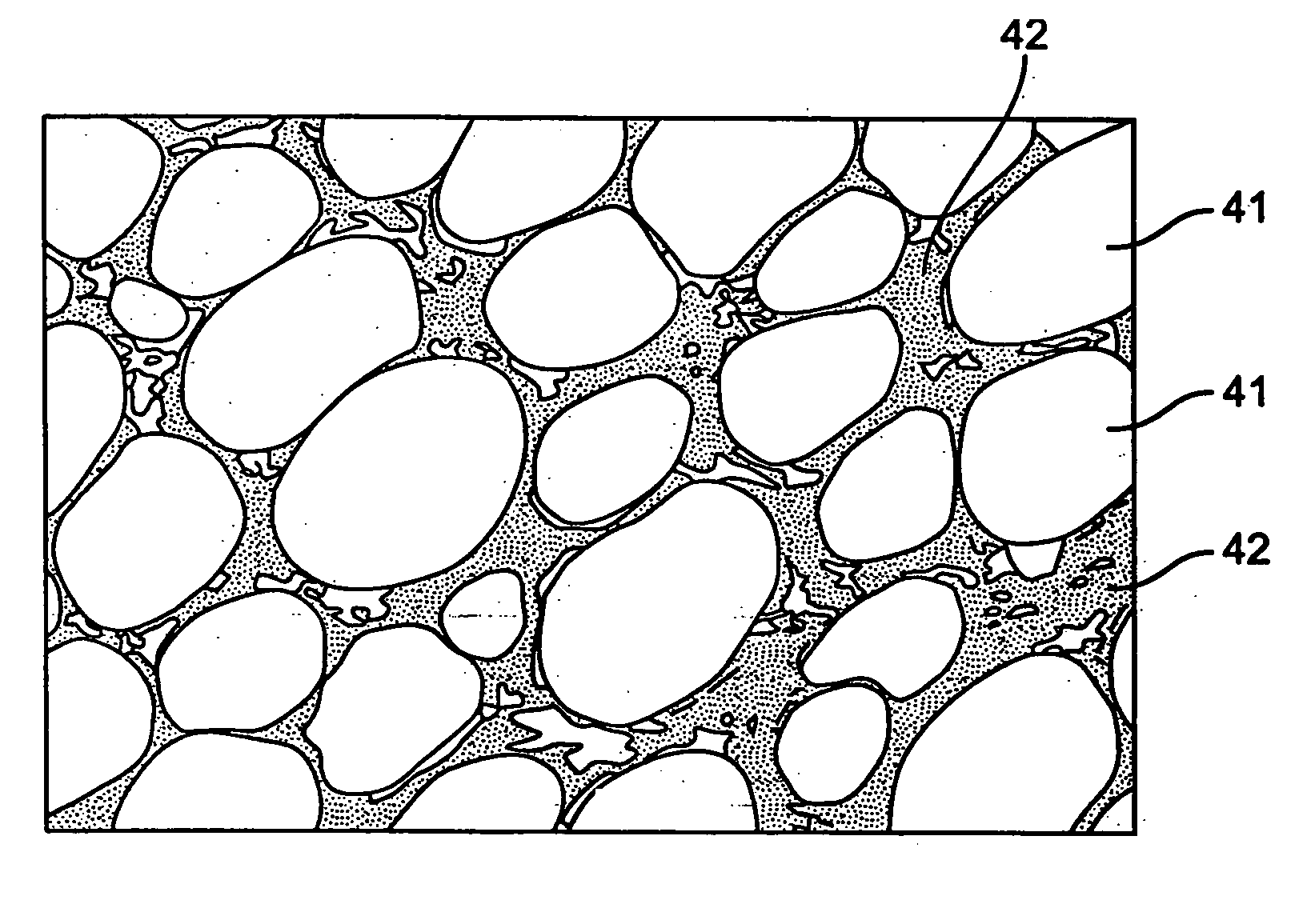
[0018] Each cell 13 may be composed of alternating positive and negative plates immersed in an electrolytic solution. The electrolytic solution composition may be chosen to correspond with a particular battery chemistry. For example., while lead acid batteries may include an electrolytic solution of sulfuric acid and distilled water, nickel-based batteries may include alkaline electrolyte solutions that include a base, such as potassium hydroxide, mixed with water. It should be noted that other acids and other bases may be used to form the electrolytic solutions of the disclosed batteries.
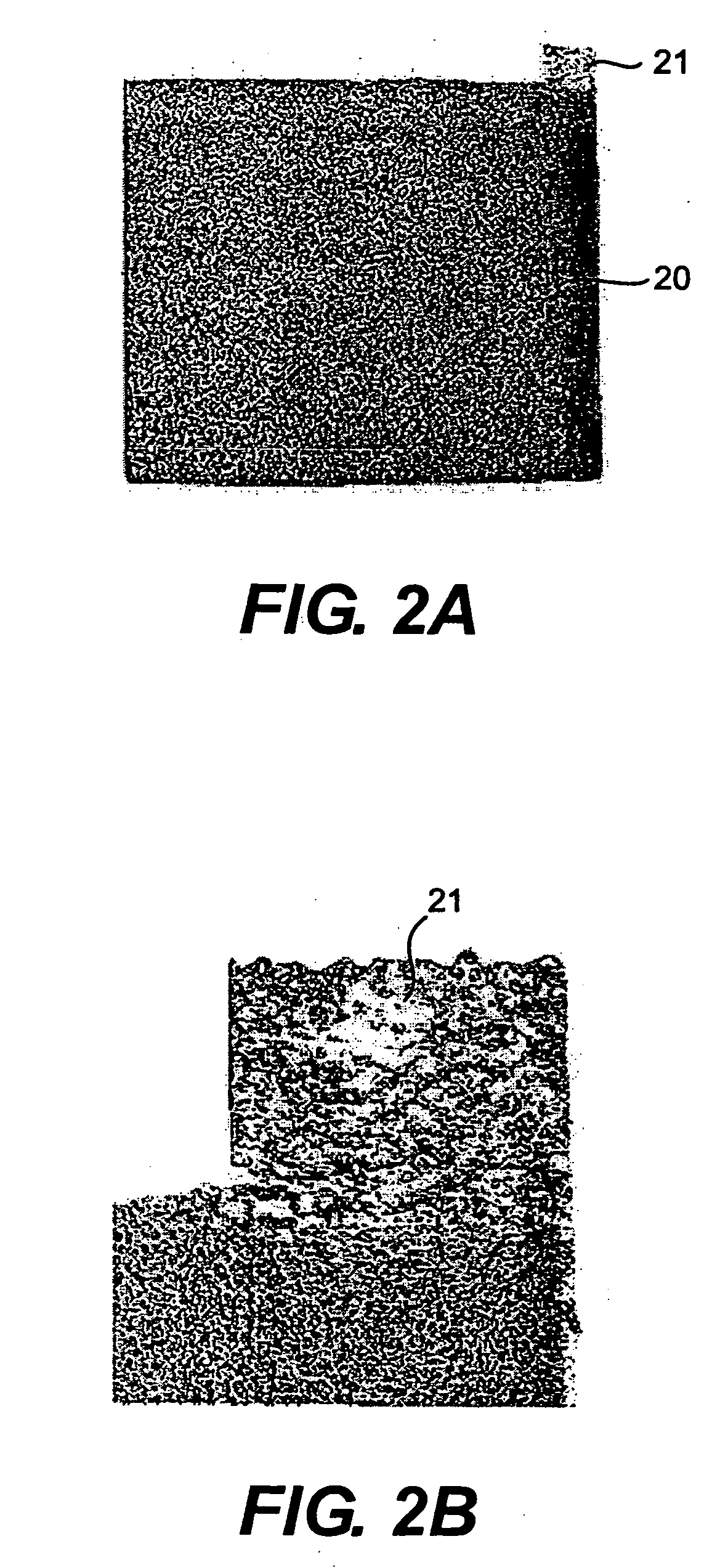
[0019] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com