Oil recovery method using alkali and alkylaryl sulfonate surfactants derived from broad distribution alpha-olefins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
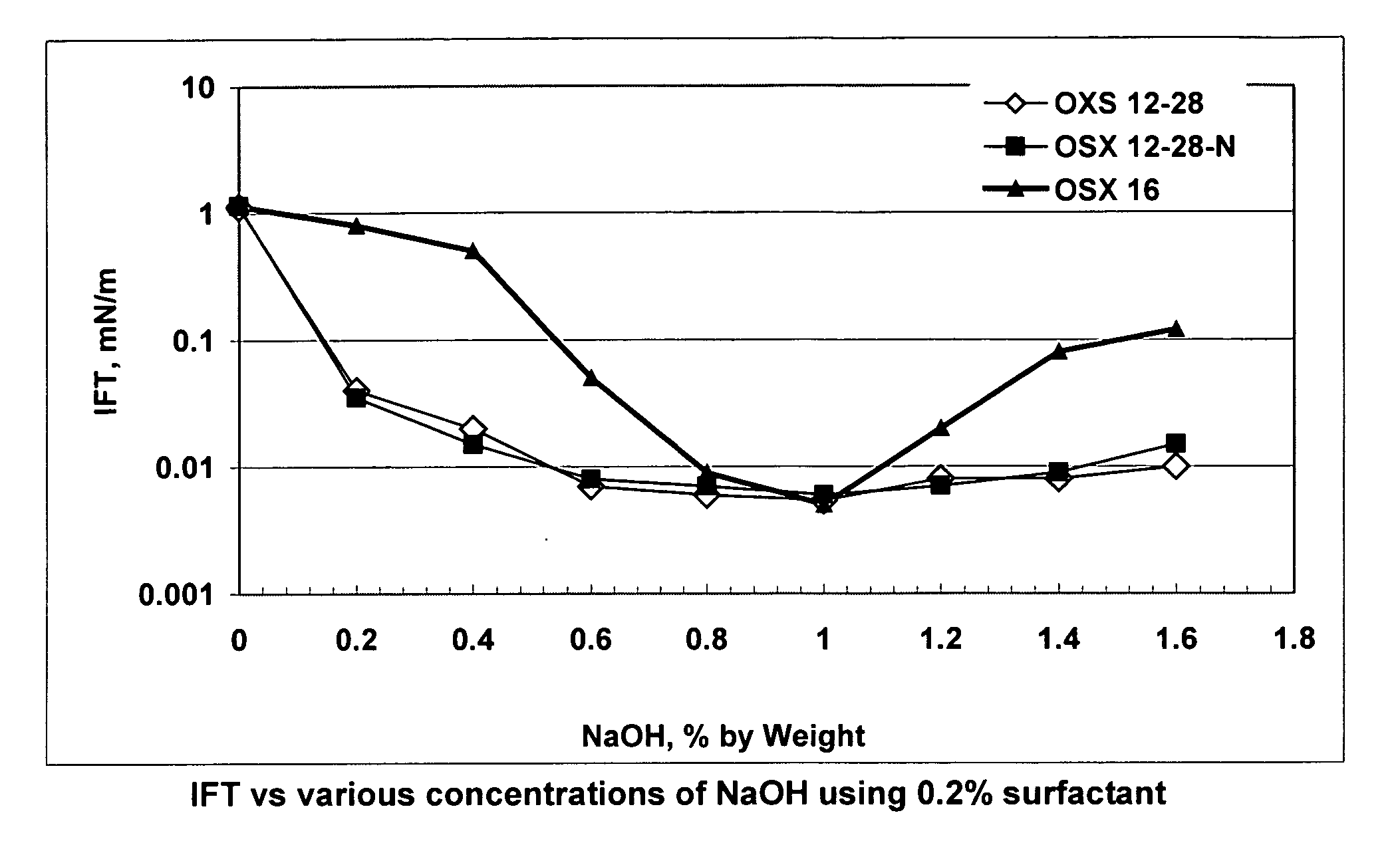
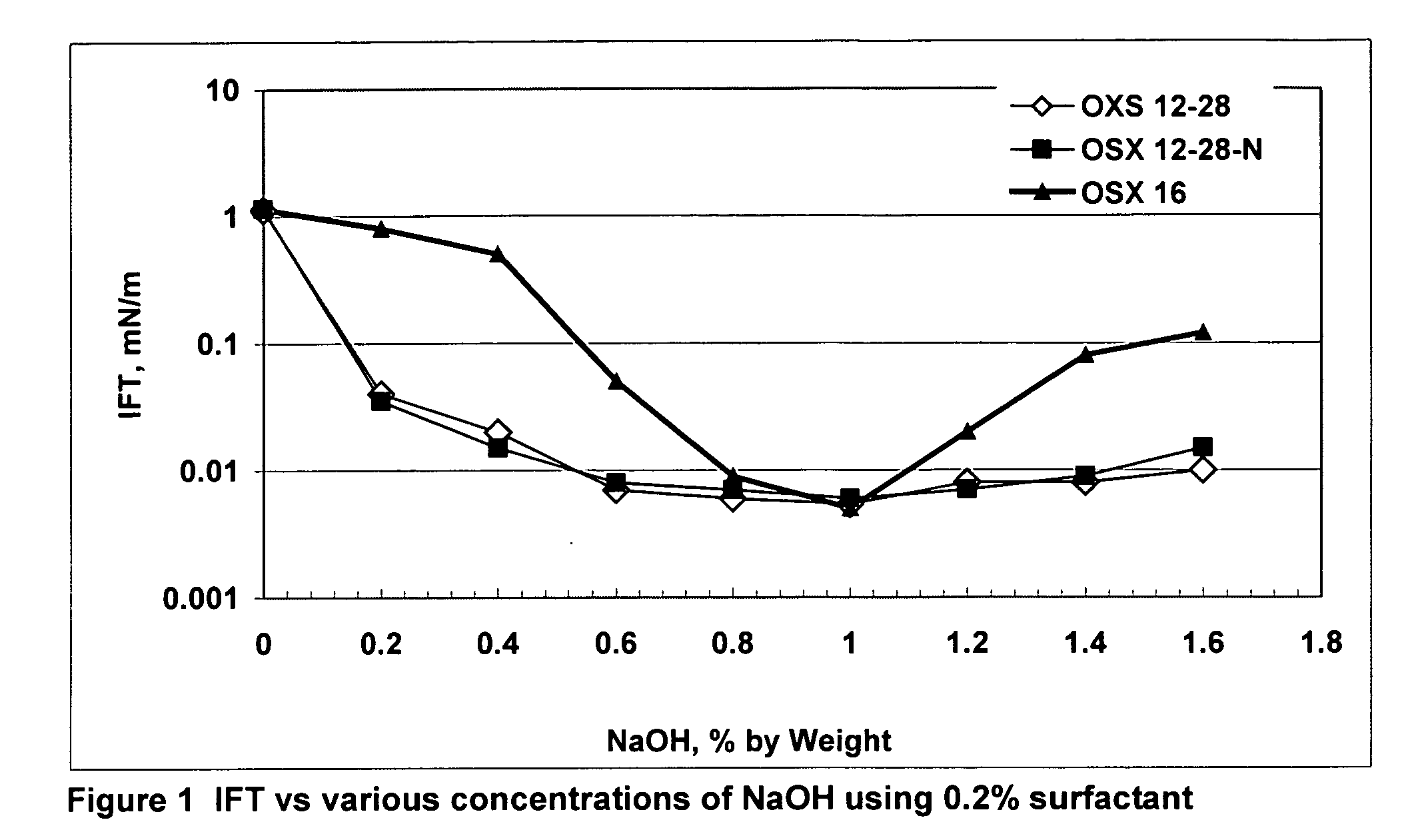
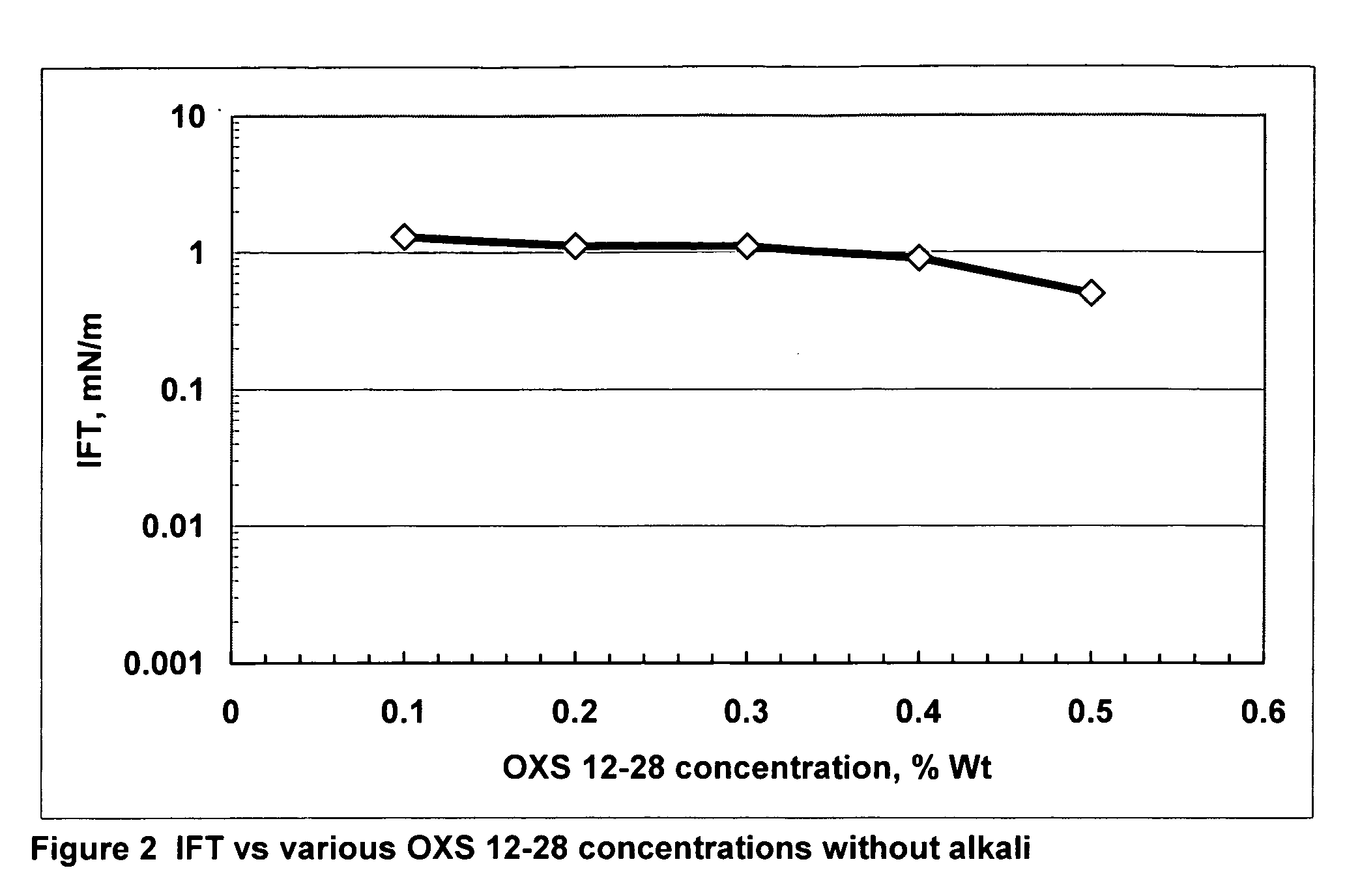
[0010] The present invention is the inclusion of alkali(s) along with alkylaryl sulfonates derived from a broad distribution of alpha-olefins greater than C10 to C58 or more, or preferably the entire C10 bottoms fraction, i.e., greater than C10 of an alpha-olefin process. Suitable ranges are C12 to C40 preferably ranges such as C10 to C32; C12 to C28; and C10 to C24. In contrast, conventionally used alkylaryl sulfonates generally focus on a narrow range of olefin carbon numbers, such as C12 xylene sulfonate, C12 benzene sulfonate, C16 xylene sulfonate, C18 toluene sulfonate, and C20-24 toluene sulfonate. In these cases, other carbon chain lengths made during the reaction must be separated out. This adds to the cost of the specifically used product. In commercial applications of alkali surfactant floods, the quantity of surfactant required is huge, often exceeding 100 million pounds lbs. If only a narrow fraction of the alpha-olefins are used to make the surfactant, the required olef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com