Anti-idiotypic antibody and its use in diagnosis and therapy in HIV-related disease
a technology of anti-idiotypic antibodies and hiv-related diseases, which is applied in the field of diagnosis and treatment of aids, can solve the problems of destroying the immune system, the true impact of the disease has yet to be felt, and the personal, social and economic impact of aids will be enormous, so as to influence the anti-idiotypic regulation of t cells and regulate the immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Isolation and Characterization of Anti-Idiotypic Antibody
1. Materials
[0064] Human polyclonal anti-HIV immunoglobulin (HIVIG), lot VH 102 was obtained from NIAID AIDS Research reference Reagent Program (ERC Bioservices Corporation, Rockville, Md.). Human pooled IgG (IVIG) was purchased from Cutter Biological, Elkhart, Ind. Normal human HIV-sera was obtained from the San Diego Regional Blood Bank. HIV positive (HIV+) sera from healthy, seropositive individuals were obtained from North American Biological Inc. (NABI), Miami, Fla.
[0065] Recombinant p24. (HTLV-IIIB) and the p24 / gp41 fusion protein were obtained from Dr. Torsten Helting, Pharmacia Genetic Engineering, La Jolla, Calif. Recombinant gp120 (HTLV IIIB), or gp120 (MN) V3 loop peptides, and recombinant HIV-1 reverse transcriptase (RT, p65) were purchased from American Biotechnologies, Inc., Cambridge, Mass. Recombinant gp120 (SF2) was obtained from Chiron Corporation, Emeryville, Calif. The antigen designations IIIB, MN, SF2,...
example ii
Clonotypic Analysis of HIV+ and HIV− Human Serum
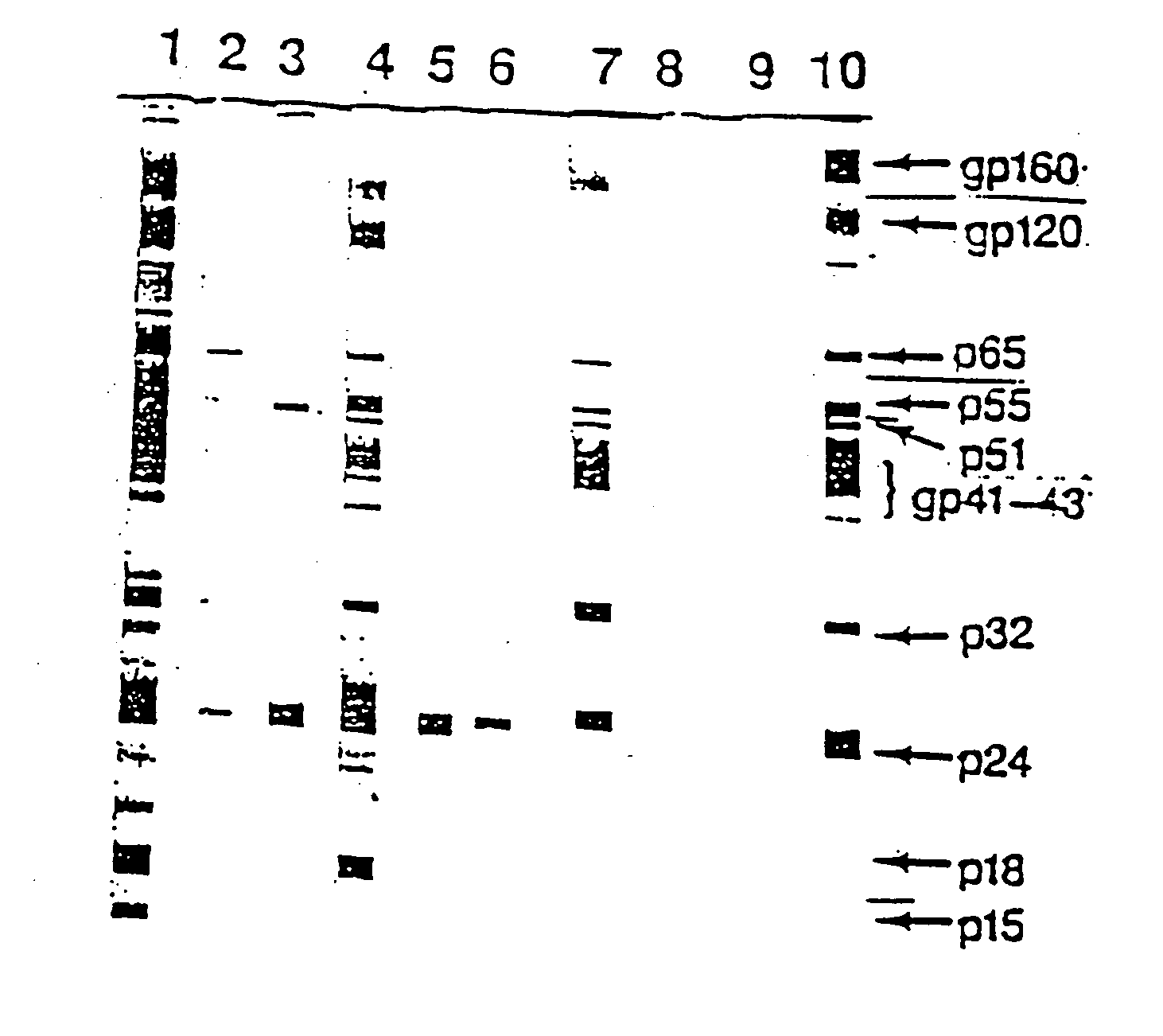
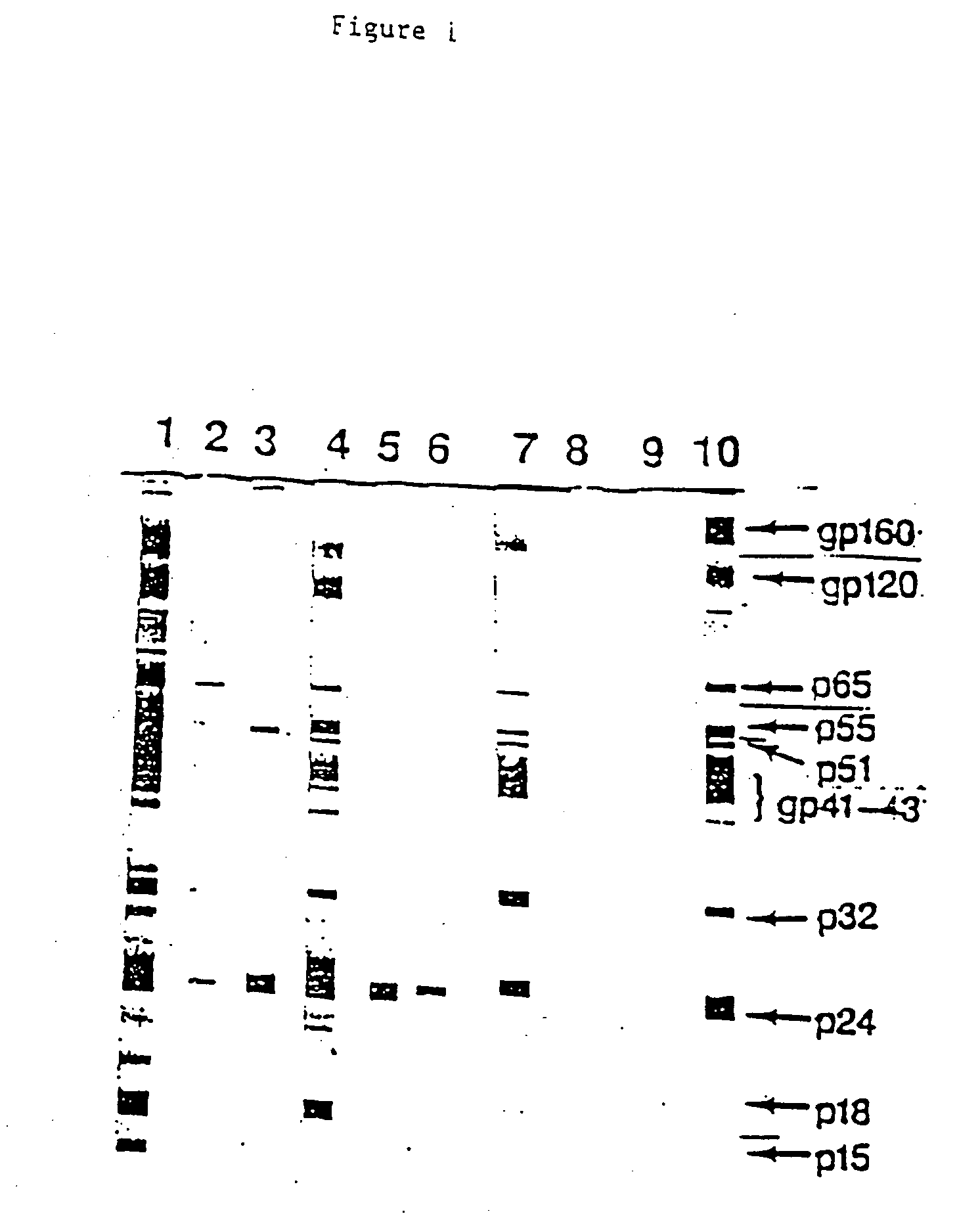
[0086] According to the Western blot show in FIG. 1 described above, three 1F7 reactivity patterns with HIV+ sera were observed. In one group, represented by #14 HIV+ serum, 1F7 reacted to antibodies to more than one HIV antigen. In the second group, represented by #3 HIV+ serum, 1F7 showed reactivity only with anti-core (p24) antibodies. Serum from the third group showed no 1F7-positive anti-HIV antibodies.
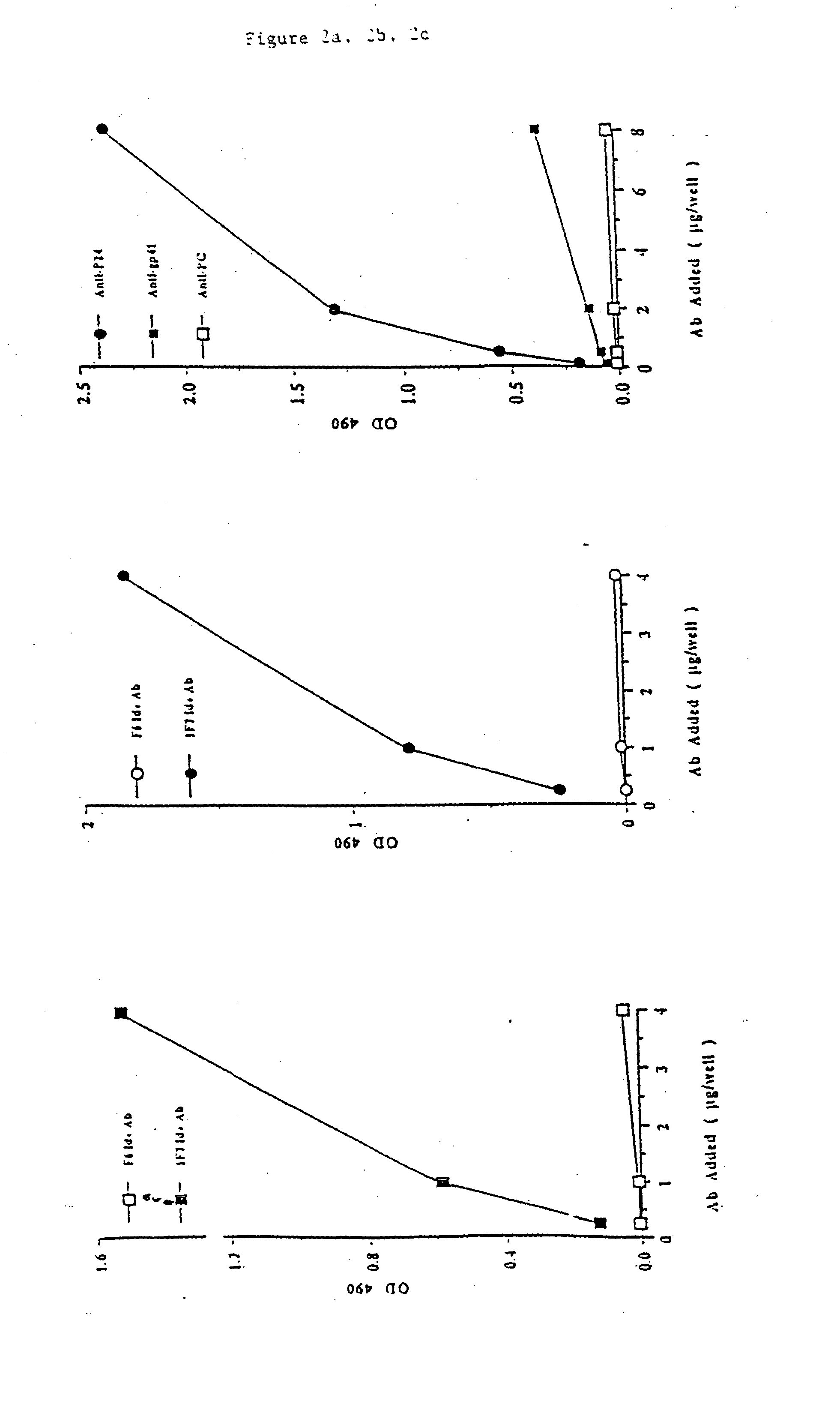
[0087] Further testing was performed in order to analyze the representation of these three groups in HIV+ sera. The detection of anti-gp120and anti-p24 antibodies, and in addition, the detection of 1F7 Id+ on antibodies against gp120and p24 in HIV+ sera was determined as follows. First, anti-HIV antibodies were detected as described above, by coating microtiter plates with 200 ng / well of gp120 or p24. A 1:40 dilution of HIV+ individual serum was added and incubated. The antibodies which bound to gp120 and p24 were determined by pe...
example iii
Evidence that Anti-HIV Antibody Responses in HIV+ Individuals are of Restricted Clonal Origin
A. Determining Clonal Restriction of B-Cell Clones by Kappa / Lambda Light Chain Analysis
1. Methods
[0092] Purification of human serum antibodies was performed by coupling recombinant p24 (HIV-1 IIIB) (Pharmacia Genetic Engineering, La Jolla, Calif.) and recombinant gp120(SF-2) (Chiron Corporation, Emeryville, Calif.) to CNBr-activated Sepharose 4B (Pharmacia LKB, Biotechnology AB, Uppsala, Sweden). According to the manufacturer, IgG from HIV+ sera (North American Biological, Inc., Miami, Fla.) was purified on protein G sepharose. The Ig fraction was passed over affinity columns at 5 ml / hour. After washing, the column was eluted using 0.1M glycine buffer (pH 2.5) with 0.01 M PBS (pH 7.0). The antibodies were dialyzed and concentrated for further use.
[0093] Detection of antibody light chain isotypes in normal (San Diego Regional Blood Bank, San Diego, Calif.) and unfractionated HIV+ human se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com