Anti-neovasculature preparations for cancer
a technology of ovasculature and preparation, which is applied in the direction of antibody medical ingredients, peptide/protein ingredients, angiogenin, etc., can solve the problems that the treatment of cancer remains challenging, and achieve the effect of rapid tumor appearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0056] A preclinical study was carried out using the already identified antigens PSMA and ED-B disclosed herein. The results of the study revealed excellent candidate epitopes. See table 9 below.
example 1.1
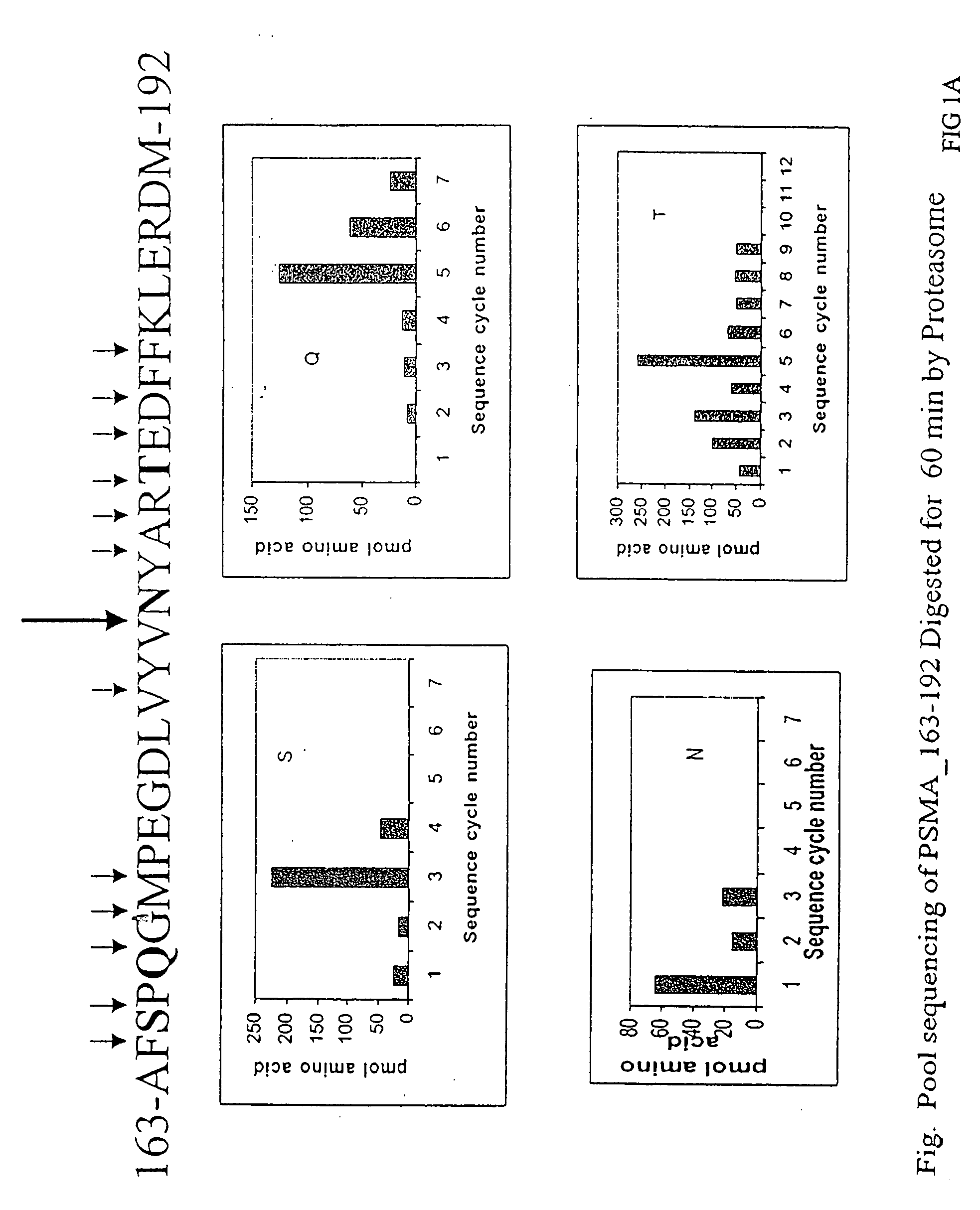
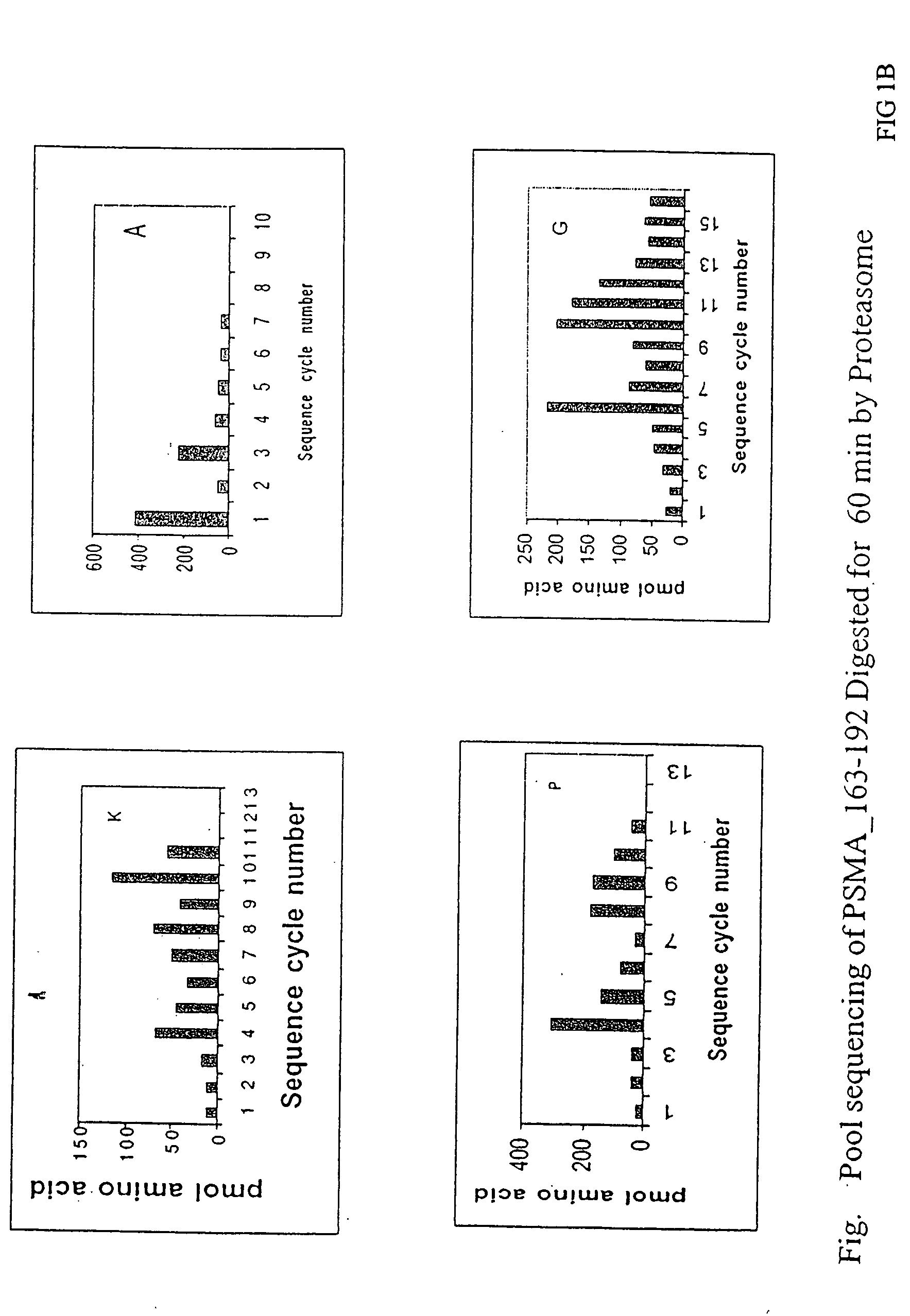
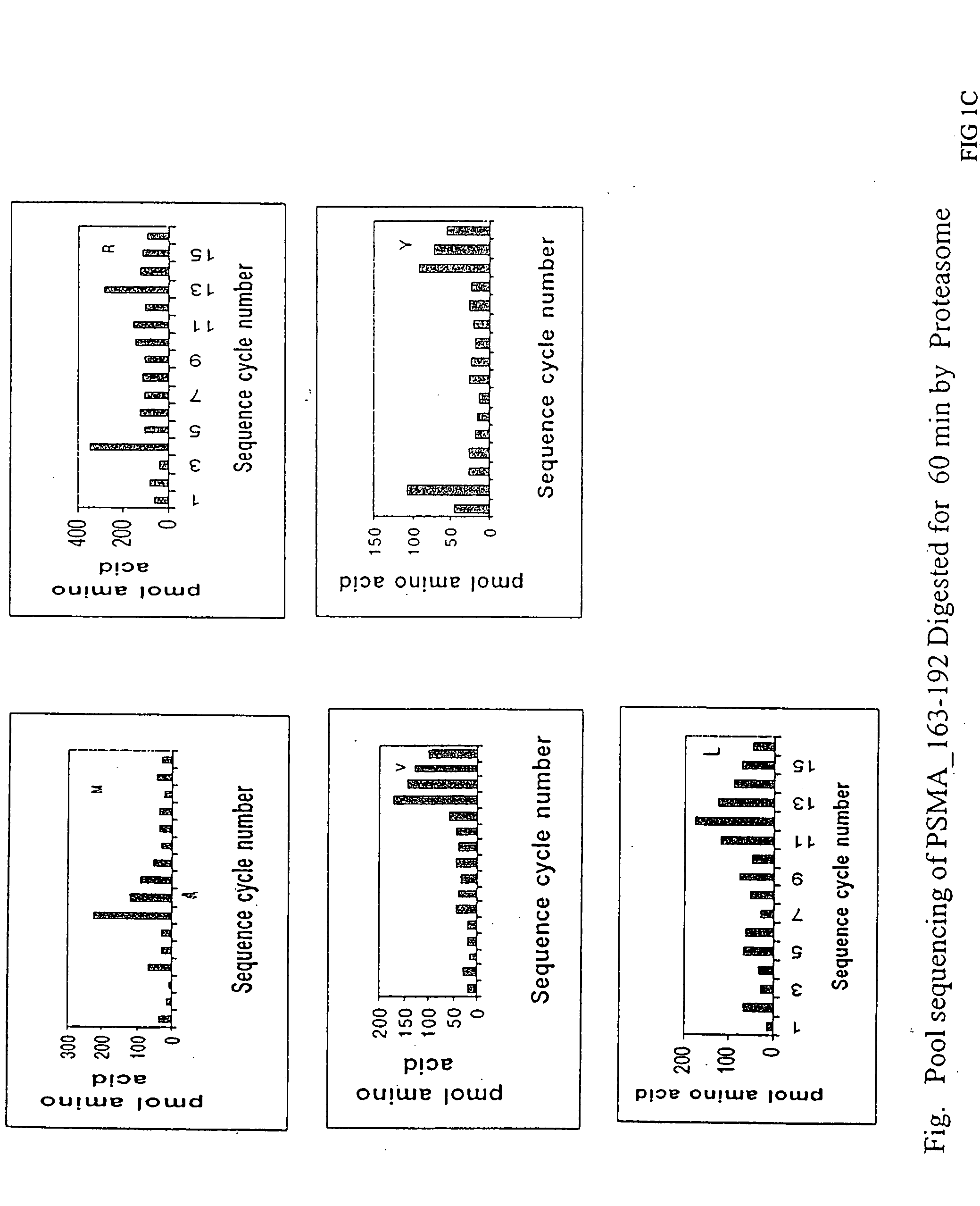
Cluster Analysis (PSMA163-192)
[0057] A peptide, AFSPQGMPEGDLVYVNYARTEDFFKLERDM, PSMA163-192, (SEQ ID NO. 3), containing an A1 epitope cluster from prostate specific membrane antigen, PSMA168-190 (SEQ ID NO. 4) was synthesized using standard solid-phase F-moc chemistry on a 433A ABI Peptide synthesizer. After side chain deprotection and cleavage from the resin, peptide first dissolved in formic acid and then diluted into 30% Acetic acid, was run on a reverse-phase preparative HPLC C4 column at following conditions: linear AB gradient (5% B / min) at a flow rate of 4 ml / min, where eluent A is 0.1% aqueous TFA and eluent B is 0.1% TFA in acetonitrile. A fraction at time 16.642 min containing the expected peptide, as judged by mass spectrometry, was pooled and lyophilized. The peptide was then subjected to proteasome digestion and mass spectrum analysis essentially as described above. Prominent peaks from the mass spectra are summarized in Table 1.
TABLE 1PSMA163-192 Mass Peak Identifi...
example 1.2
Cluster Analysis (PSMA281-310).
[0082] Another peptide, RGIAEAVGLPSIPVHPIGYYDAQKLLEKMG, PSMA281-310, (SEQ ID NO. 18), containing an A1 epitope cluster from prostate specific membrane antigen, PSMA283-307 (SEQ ID NO. 19), was synthesized using standard solid-phase F-moc chemistry on a 433A ABI Peptide synthesizer. After side chain deprotection and cleavage from the resin, peptide in ddH2O was run on a reverse-phase preparative HPLC C18 column at following conditions: linear AB gradient (5% B / min) at a flow rate of 4 ml / min, where eluent A is 0.1% aqueous TFA and eluent B is 0.1% TFA in acetonitrile. A fraction at time 17.061 min containing the expected peptide as judged by mass spectrometry, was pooled and lyophilized. The peptide was then subjected to proteasome digestion and mass spectrum analysis essentially as described above. Prominent peaks from the mass spectra are summarized in Table 3.
TABLE 3PSMA281-310 Mass Peak Identification.CALCU-SEQLATEDIDMASSNO.PEPTIDESEQUENCE(MH+)1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com