Method for carrying out a biochemical protocol in continuous flow in a microreactor
a microreactor and biochemical technology, applied in the direction of fluid speed measurement, optical light guide, water/sand/air bath, etc., can solve the problems of not allowing large-scale protocols to be performed, affecting the flow rate of the microreactor, so as to reduce the number of distributions, improve the throughput, and reduce the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PCR Reaction in a Continuous Flow in a Microfluidic Device
[0370] A PCR reaction mixture was run through a channel in which a PCR was performed.
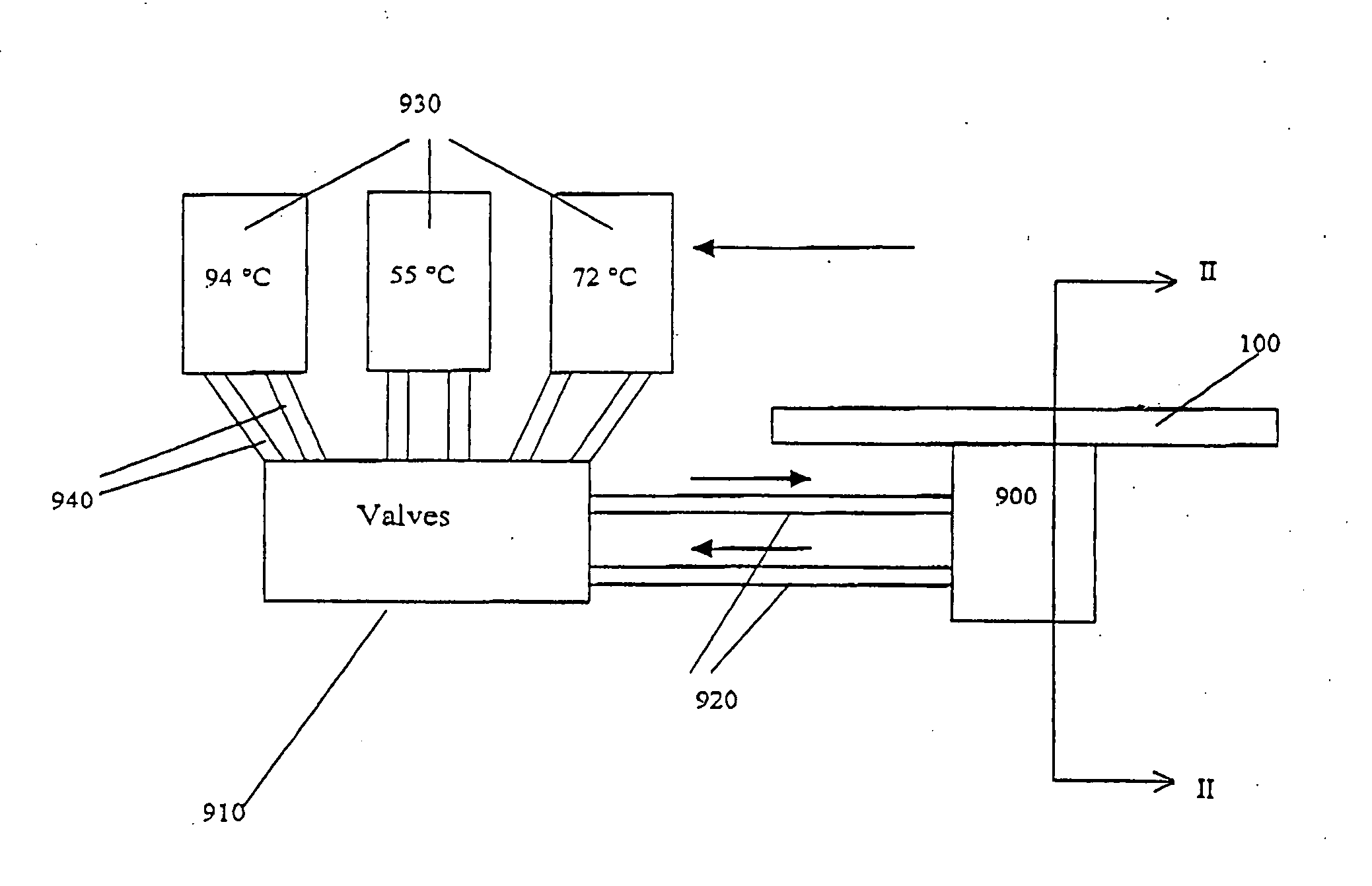
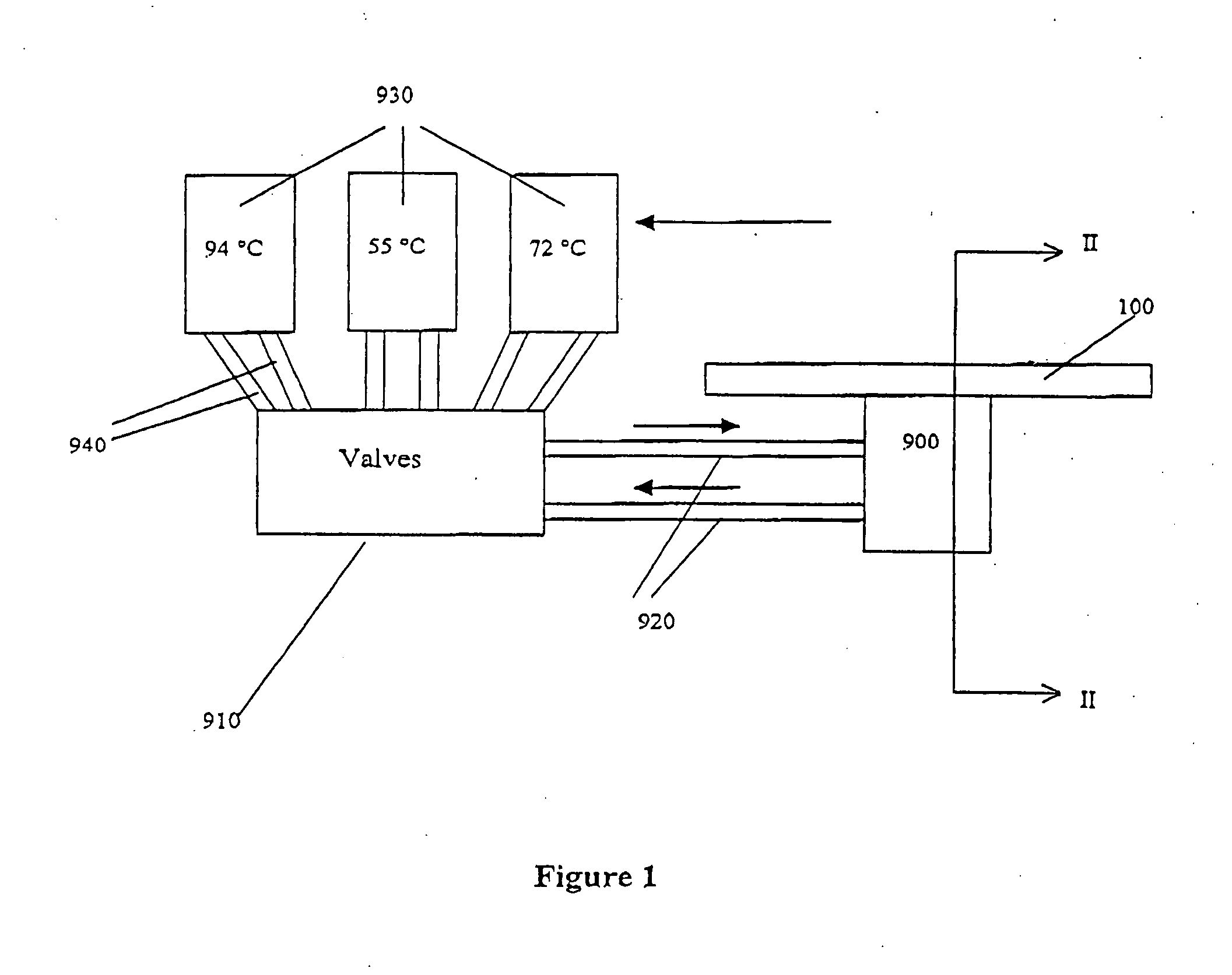
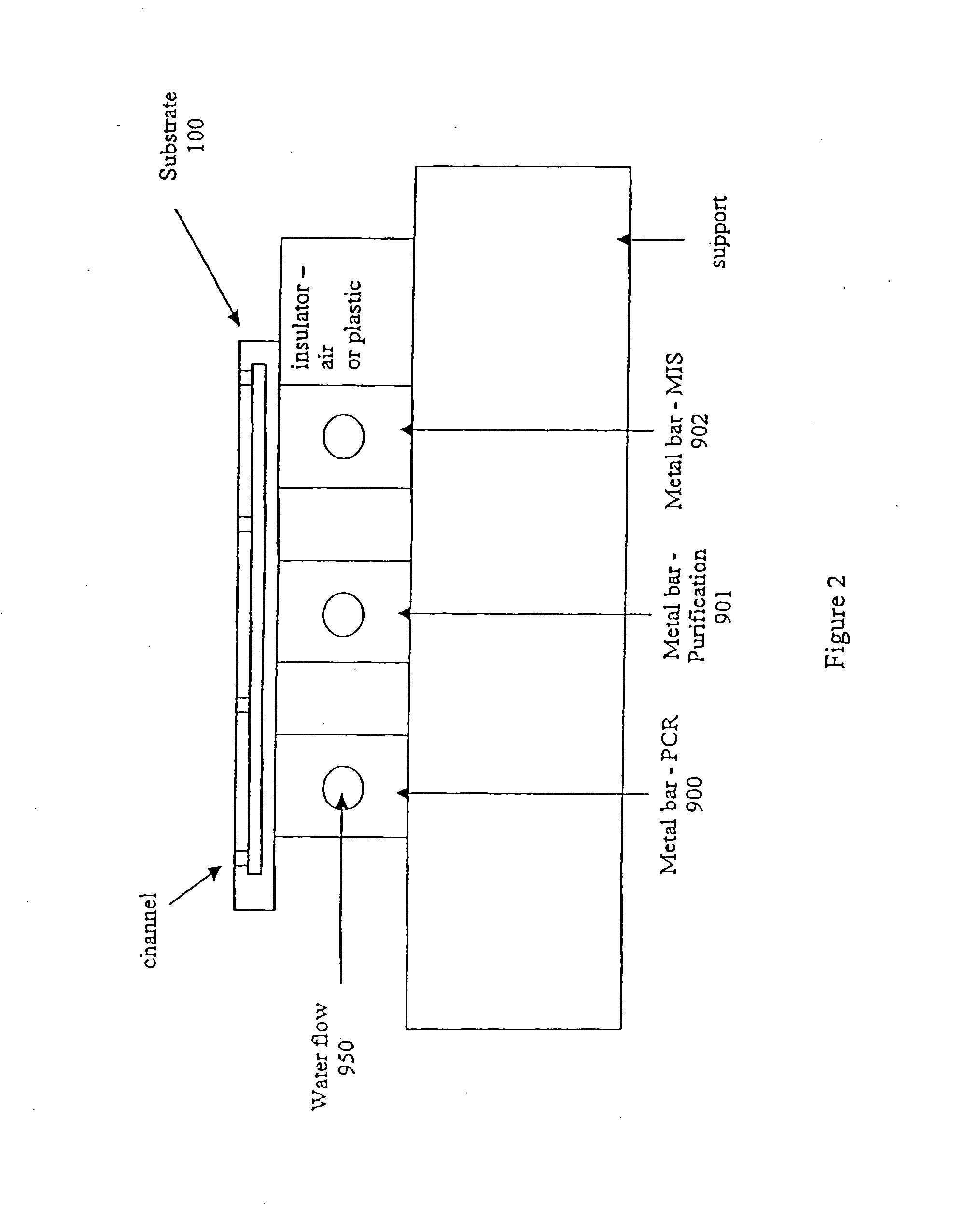
[0371] The microfluidic substrate comprised 10 channels in parallel chemically etched in silicone. The channels were rectilinear with a diameter of the order of about 600 μm in the reaction zones. The surface area of the section of a channel was of the order of about 0.25 mm2.
[0372] The PCR was carried out in parallel in 3 channels. The volume of one PCR reaction was 1.2 μl, but ten identical PCR reactions (12 μl (10×1.2 μl)) were performed for out of the device post PCR sample analysis (quantification and size analysis).
[0373] The microfluidic substrate was siliconized just before the substrate was used. Before the PCR reaction, all the channels were filled with previously degassed and filtered water. A high flow rate of the order of 25 μl / min was applied for 15 minutes to remove all the air bubbles present in the circuits. A previously ...
example 2
Integration of a Genotyping Protocol in a Continuous Flow Microfluidic Device
[0379] In one embodiment, the reaction mixture runs through a channel in which all the steps required for a genotyping protocol are performed: PCR, purification, microsequencing reaction and detection.
[0380] The microfluidic substrate comprises 100 channels in parallel. These channels are rectilinear and have a diameter of the order of 600 μm in the reaction zones. The surface area of the section of a channel is of the order of 0.25 mm2.
[0381] The genotyping protocol is carried out in parallel in the 100 channels. 100 samples are injected in parallel into the channels, and 100 such injections of sample are carried out sequentially, so that each channel contains 100 injections of the same sample. The microfluidic substrate thus makes it possible, by means of a cross-distribution of 100 samples for 100 polymorphisms, to carry out 10,000 genotyping reactions on a microfluidic substrate.
[0382] The injection...
example 3
Genotyping by the Method of Allele-Specific Ligase Chain Reaction (LCR)
[0399] Allele-specific LCR, as disclosed in Barany et al. (PCR Meth. Appl. 1: 5-16 (1991), the contents of which are incorporated herein by reference in its entirety), employs four oligonucleotides two of which hybridize to one strand of target DNA and a complementary set of adjacent oligonucleotides, which hybridize to the opposite strand. Thermostable DNA ligase will covalently link each set, provided there is complete complementarity at the junction. A single-base mismatch at the oligonucleotide junction will not be amplified and is therefore distinguished; a second set of mutant-specific oligonucleotides is used in a separate reaction to detect or confirm the mutant allele(s).
[0400] A homogeneous phase protocol for allele-specific LCR can be carried out in accordance with the present invention by introducing into each channel 10 μl of starting mixture comprising: the DNA with the target sequence to be analy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com