Healing transmyocardial implant
a transmyocardial implant and implant technology, applied in the field of implants, can solve the problems of not facilitating full healing and achieve the effect of promoting healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
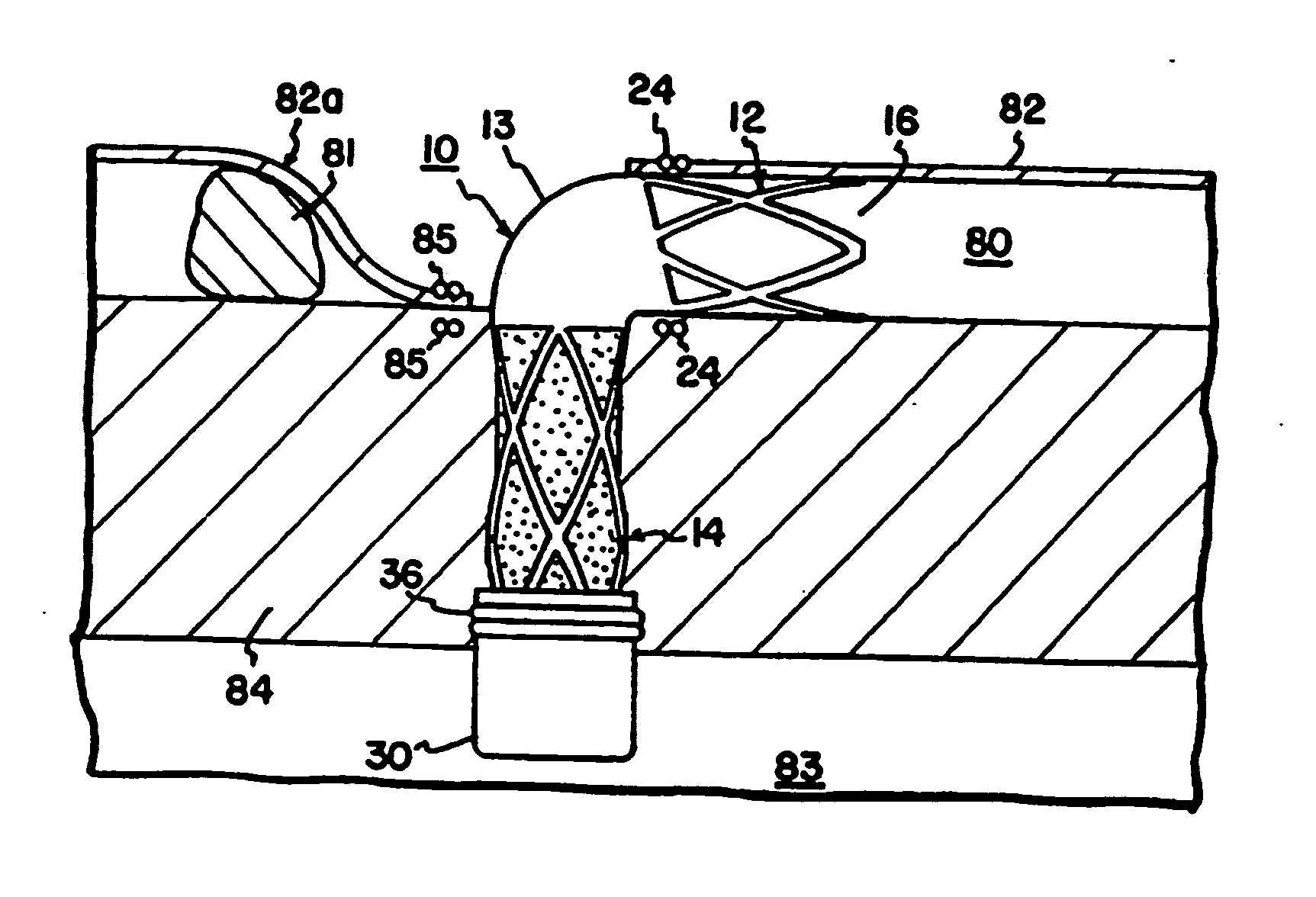
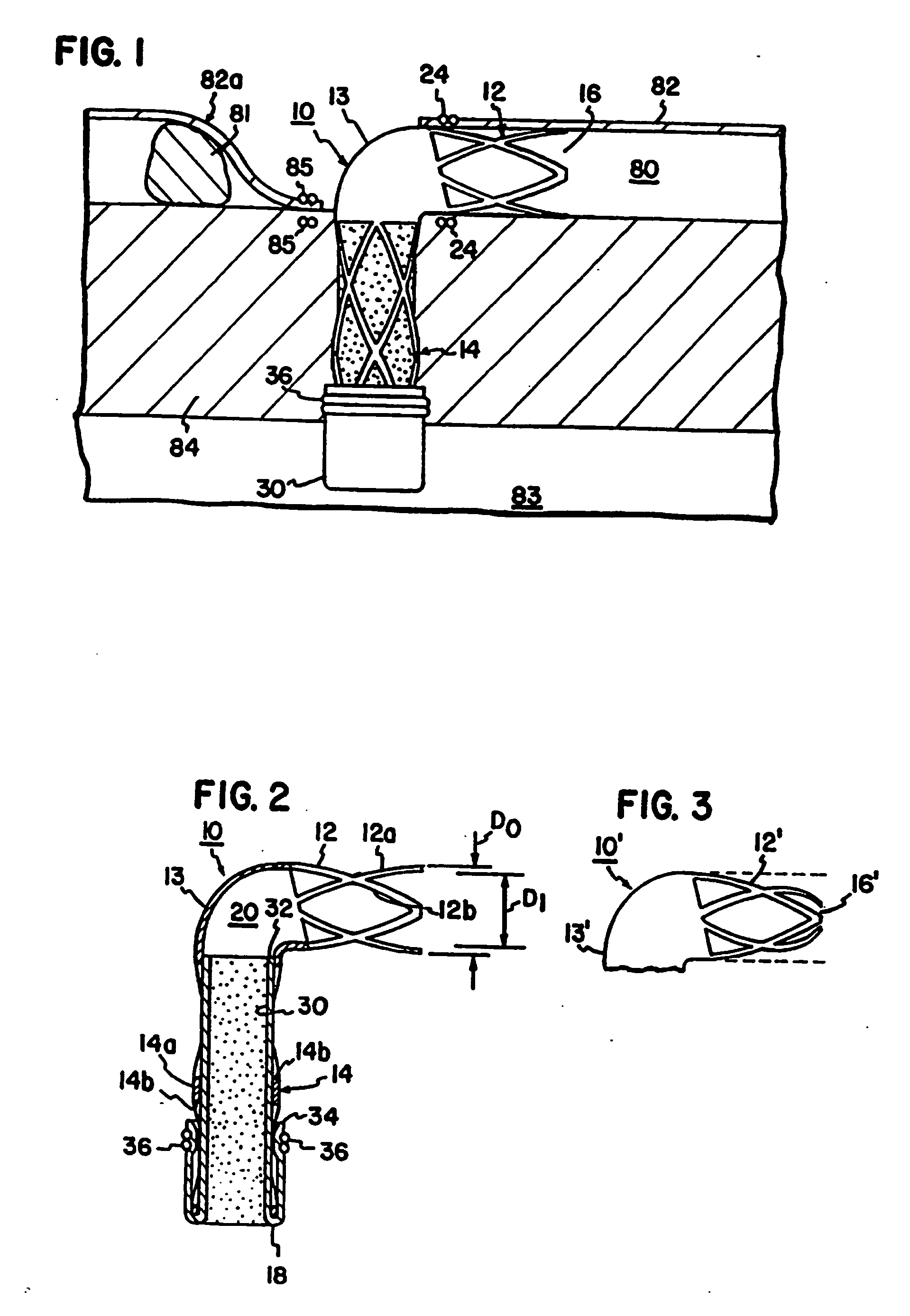
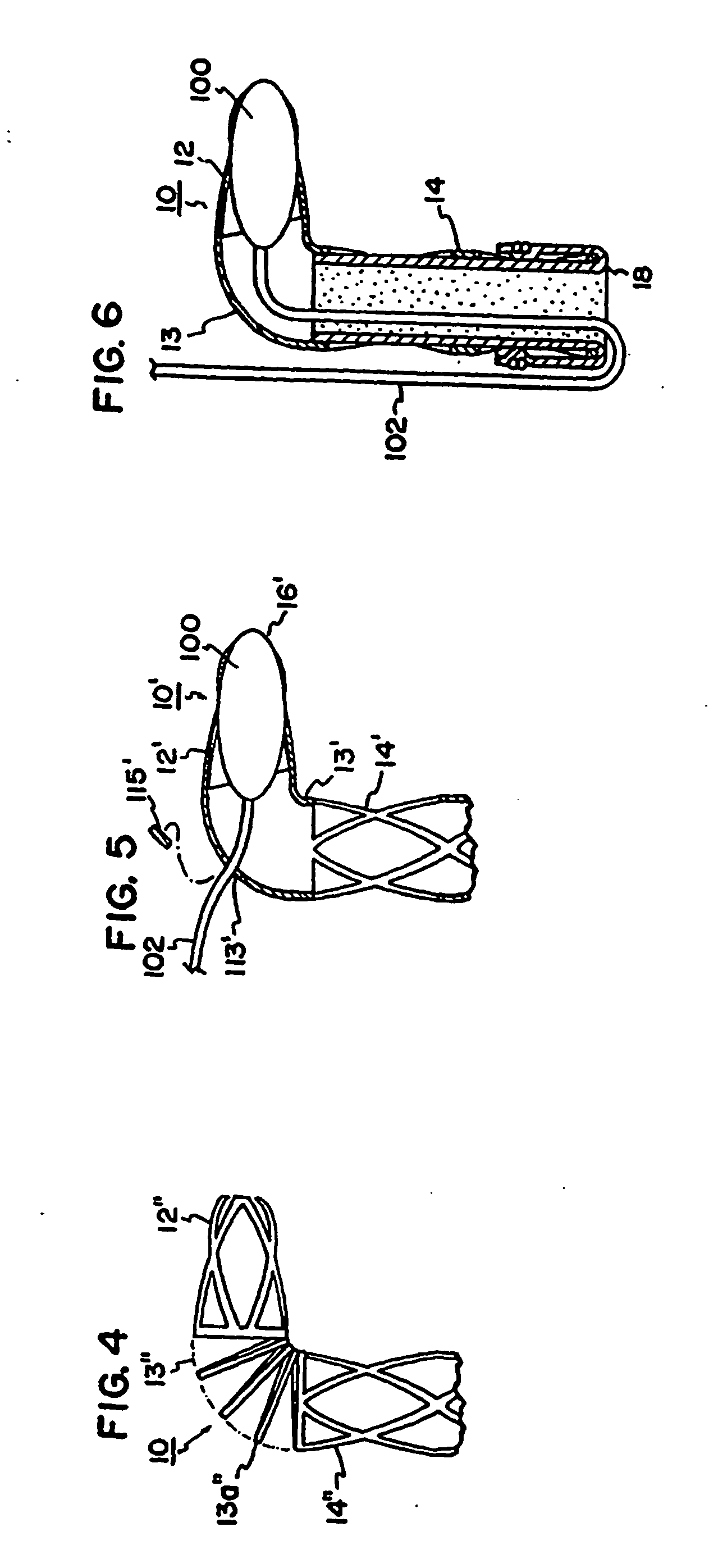
[0016] With initial reference to FIG. 1, a conduit 10 is shown in the form of an L-shaped tube. The conduit 10 may be formed of titanium or other biocompatible material. The material of the conduit 10 is preferably radially rigid material in order to withstand contraction forces of the myocardium. By way of non-limiting example, the tube will have an outside diameter D0 of about 3 millimeters and an internal diameter D1 of about 2.5 millimeters to provide a wall thickness of about 0.25 millimeters.
[0017] The tube 10 has a coronary portion 12 sized to be received within the lumen of a coronary vessel such as the lumen 80 of a coronary artery 82 distal to an obstruction 81 as illustrated in FIG. 1. The conduit 10 has a myocardial portion 14 extending at a right angle to the axis of portion 12. The myocardial portion 14 is sized to extend from the coronary artery 82 directly through the myocardium 84 and protrude into the left ventricle 83 of a patient's heart.
[0018] The coronary por...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| internal diameter D1 | aaaaa | aaaaa |
| internal diameter D1 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com