Method for the positive selection of chromosomal mutations in C1 metabolizing bacteria via homologous recombination
a chromosomal mutation and metabolizing bacteria technology, applied in the field of molecular biology, can solve the problems of low product yield, low economic success of scp, and slight commercial success of epoxidation of alkenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Growth of Methylomonas 16a
[0224] Example 1 summarizes the standard conditions used for growth of Methylomonas sp. 16a (ATCC# PTA-2402), as described in U.S. Pat. No. 6,689,601.
Methylomonas Strain and Culture Media
[0225] The growth conditions described below were used throughout the experimental Examples for treatment of Methylomonas 16a, unless conditions were specifically described otherwise.
[0226]Methylomonas 16a is typically grown in serum stoppered Wheaton bottles (Wheaton Scientific, Wheaton, Ill.) using a gas / liquid ratio of at least 8:1 (i.e., 20 mL of Nitrate liquid “BTZ” media in 160 mL total volume). The standard gas phase for cultivation contained 25% methane in air, although methane concentrations can vary ranging from about 5-50% by volume of the culture headspace. These conditions comprise growth conditions and the cells are referred to as growing cells. In all cases, the cultures were grown at 30° C. with constant shaking in a Lab-Line rotary shaker unless otherwi...
example 2
Construction of a Positive-Selective Suicide Vector for Methylomonas sp. 16a
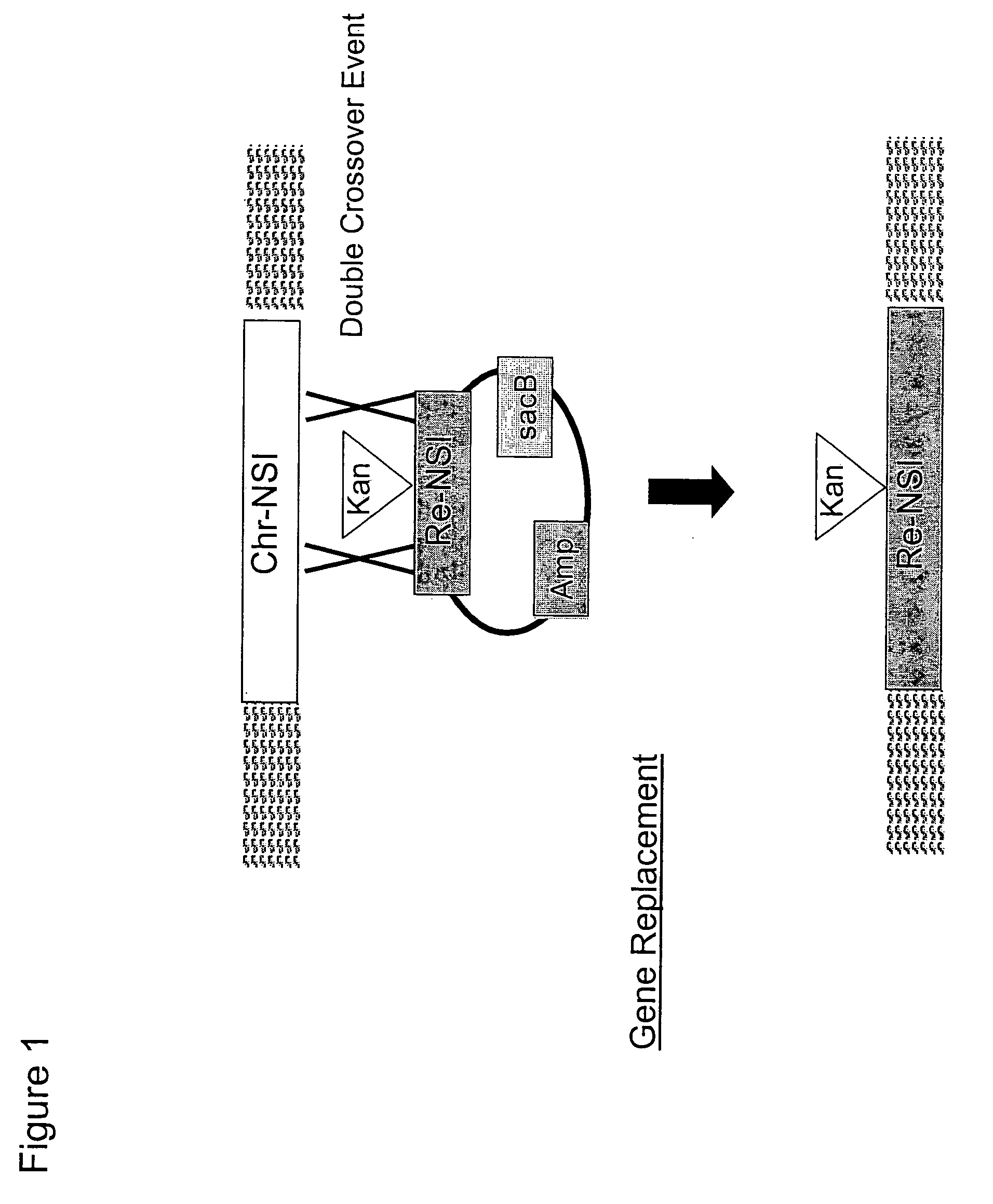
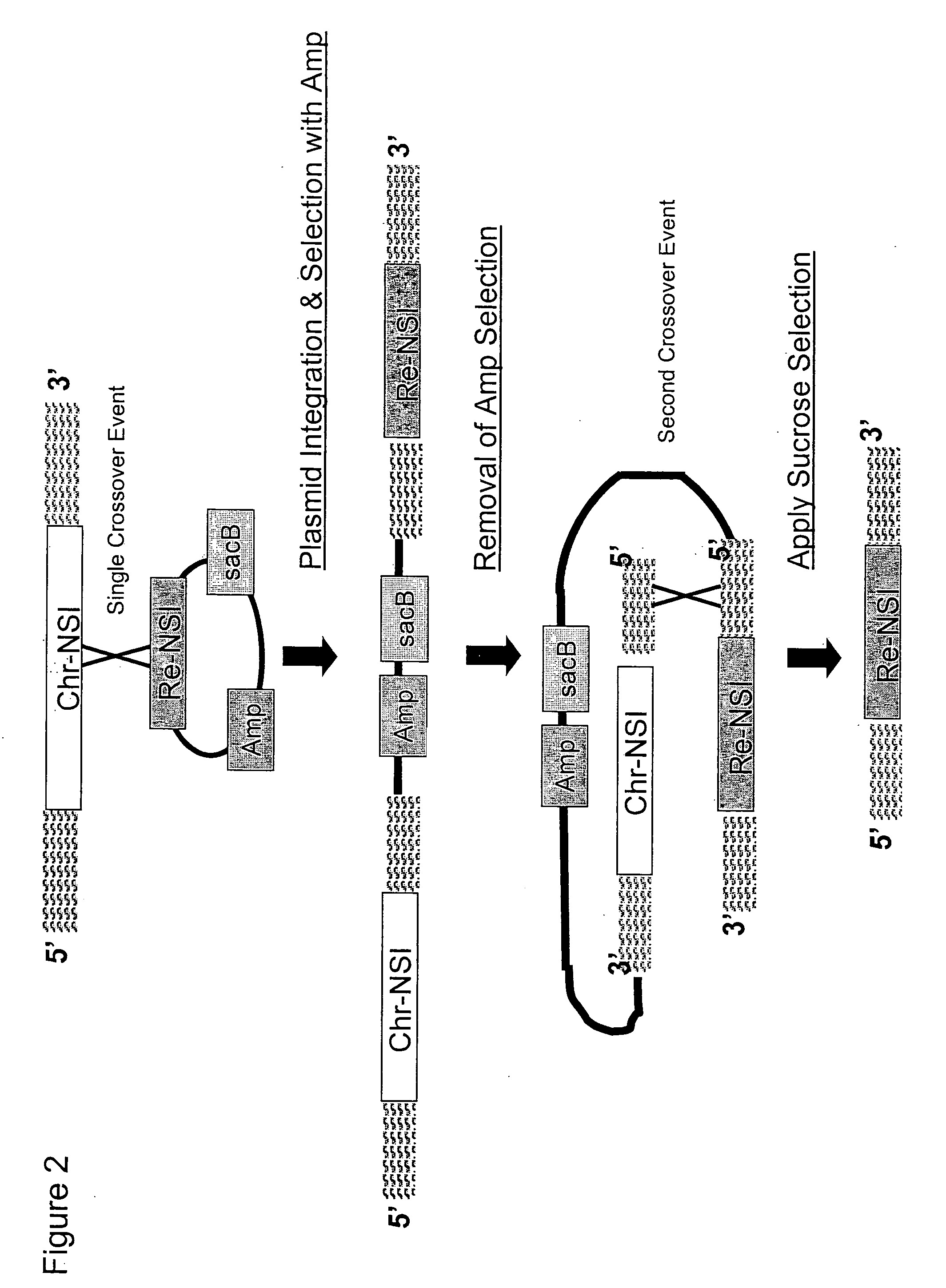
[0230] The construction of chromosomal mutations within the Methylomonas genome required the use of suicide vectors. Thus, a modified version of the conditional replication vector pGP704 was created, comprising a npr-sacB cassette.
pGP704 as a Vector Backbone for the C1 Chromosomal Integration Vector
[0231] The plasmid pGP704 (Miller and Mekalanos, J. Bacteriol., (170): 2575-2583 (1988); FIG. 4) was chosen as a suitable vector backbone for the C1 chromosomal integration vector, since it could be used as a vehicle to transfer replacement nucleotide sequences of interest into Methylomonas sp. 16a via conjugation. Plasmid pGP704 is a derivative of pBR322 that is AmPR but has a deletion of the pBR322 origin of replication (oriE1). Instead, the plasmid contains a cloned fragment containing the origin of replication of plasmid R6K. The R6K origin of replication (oriR6K) requires the Π protein, encoded by the pir...
example 3
Construction of pGP704::sacB::crtN2::EZ::TN™
[0244] The native Methylomonas gene encoding crtN2 was disrupted by EZ::TN™ (Epicenter Technologies, Madison, Wis.) to generate an appropriate replacement nucleotide sequence of interest (i.e., re-NSI) in the chromosomal integration vector pGP704::sacB. This construct, pGP704::sacB::crtN2::EZ::TN™ was then utilized in Examples 4 and 5 to test the homologous recombination abilities of Methylomonas sp. 16a, using the one-step selection strategy to identify allelic exchange mutants.
PCR Amplification and Cloning of the crtN2 DNA Fragment into pGP704::sacB
[0245] DNA primers crtN#2-ctg 288+1 kB / Bg / ll and crtN#2-ctg 288+1kB / Xbal were used to amplify a ˜3.1 kB DNA fragment from Methylomonas sp. 16a chromosomal DNA, comprising SEQ ID NO:3 flanked by approximately 1 Kb of DNA.
crtN#2-ctg 288 + 1kB / Bg / II:5′-AGATCTTTCCGGTCATGCTGGAGTTGGG-3′(SEQ ID NO:6)crtN#2-ctg 288 + 1kB / XbaI:5′-TCTAGATTGCAGCTCAAGCGATTCGG-3′(SEQ ID NO:7)
[0246] As described in Exa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com