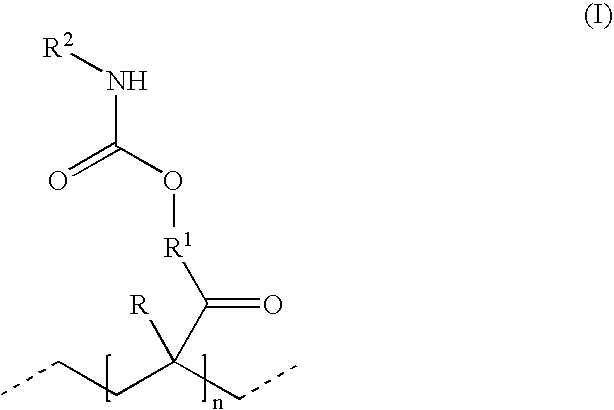
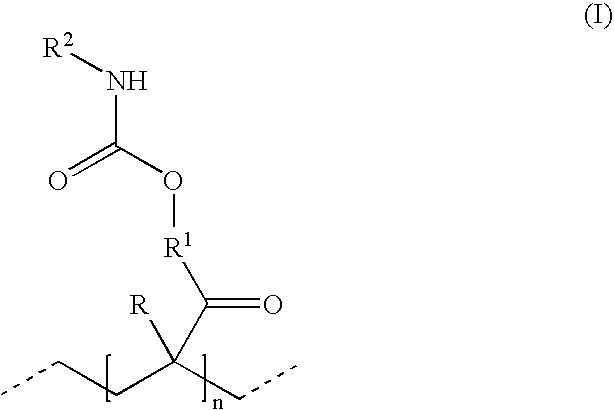
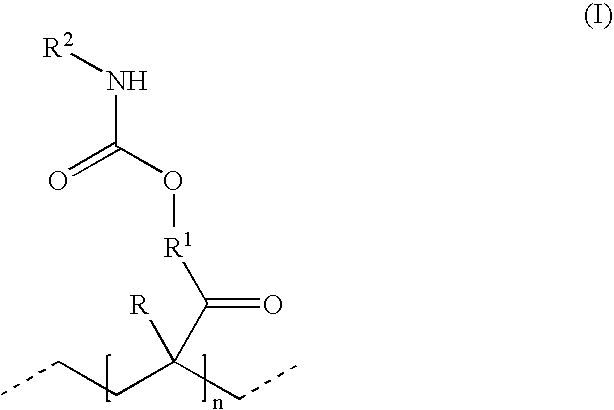
Polyisocyanate mixtures, a process for their preparation and their use in coating compositions
a technology of polyisocyanate and mixture, which is applied in the field of modified polyisocyanate mixture, can solve the problems of merely moderate acid resistance, scratch resistance and the general inadequate reflow of such coating composition, and achieves the effects of reducing the number of reflows, and improving the reflow ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0065] Abbreviations and ingredients used:
[0066] HEA: Hydroxyethyl acrylate
[0067] HEMA: Hydroxyethyl methacrylate
[0068] HPMA: Hydroxypropyl methacrylate
[0069] Desmodur® HL BA: Aromatic-aliphatic polyisocyanate based on toluene diisocyanate / hexamethylene diisocyanate (HDI), 60% in butyl acetate, NCO content 10.5%, available from Bayer MaterialScience AG, Leverkusen DE.
[0070] Desmodur® IL BA: Aromatic polyisocyanate based on toluene diisocyanate, 51% in butyl acetate, NCO content 8.0%, available from Bayer MaterialScience AG, Leverkusen DE.
[0071] Desmoduro® 3200: Aliphatic, biuret group-containing polyisocyanate based on HDI, solvent-free, NCO content 23.0%, available from Bayer MaterialScience AG, Leverkusen DE.
[0072] Desmodur® N 3300: Isocyanurate group-containing polyisocyanate based on HDI, solvent-free, NCO content 21.8%, available from Bayer MaterialScience AG, Leverkusen DE.
[0073] Desmoduro® N 3600: Low viscosity, isocyanurate group-containing polyisocyanate based on HD...
use examples
[0105] These examples describe the preparation of ready-to-use coating compositions based on the polyisocyanates PIC in comparison with the corresponding non-polyacrylate-modified starting polyisocyanates, the application of these coating compositions, and the testing of the resulting coating films.
[0106] The general coating properties were assessed by preparing transparent varnishes. For that purpose the polyisocyanates were each combined with a polyol at an NCO / OH equivalent ratio of 1:1. The polyol used was Desmophen® A 870, a polyacrylate polyol available from Bayer MaterialScience AG, Leverkusen, DE, which has a solids content of 70% by weight in butyl acetate, a viscosity of 3500 mPa·s at 23° C., an acid number of 7.5 mg KOH / g (based on as-supplied form) and an OH content of 2.95% by weight (based on as-supplied form). Based on resin solids (sum of the solid fractions of polyol and polyisocyanate) the following amounts of additives were used.
% by weight,Constituentssolids o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com