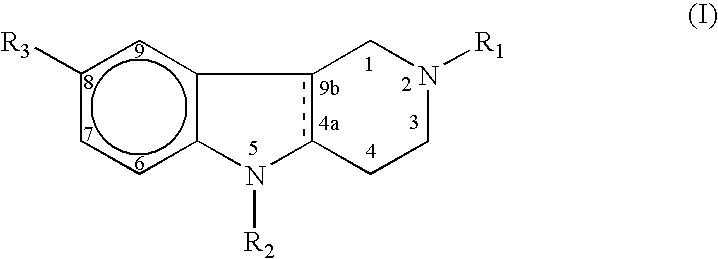
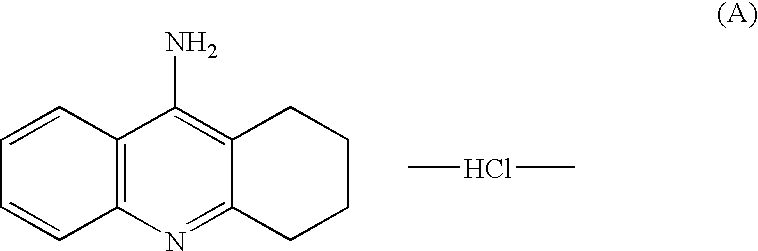
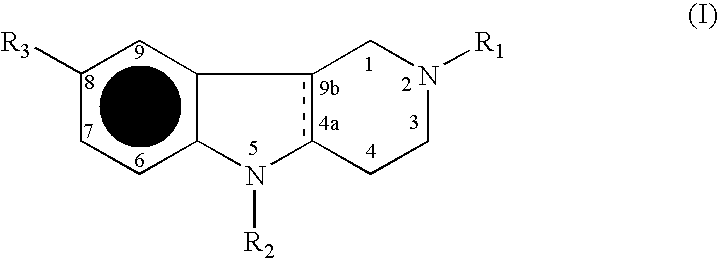
Agents for treating neurodegenerative disorders
a neurodegenerative disorder and agent technology, applied in the field of chemical compounds in medicine, can solve the problems of tacrine side effects, death, and ineffective treatment with tacrine, and achieve the effects of reducing the number of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
I. Anti-NMDA Activity of the Compounds Provided by the Present Invention
[0060] The experiment was conducted in white non-inbred male mice of 20-24 g of weight. A solution of the test agent in 0.2 ml of 5% aqueous dimethylsulfoxide was injected intraperitoneally 40 minutes before the injection of NMDA into the lateral vehicle of the brain. For the mice that had been prepared beforehand for the experiment a skin flap on the head of each mouse was removed under ether anestesia and a hole was bored in the skull by a fine drill. NMDA (0.1 μg) in a volume of 1.4 μl was injected with a microsyringe. The microsyringe needle was immersed to a depth of 2.5 mm. After the operation, the wound was treated with a 2% novocaine solution. After recovery from the anestesia, the mice showed signs of pain disturbance. The accuracy of NMDA injection was monitored by injection of methylene blue. Two to 4 hours after the operation, the mice were used for a pharmacological experiment.
[0061] The animals ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com