Novel diagnostic marker, a diagnostic kit and a method for diagnosis of rheumatoid arthritis
a diagnostic kit and rheumatoid arthritis technology, applied in the field of new diagnostic markers, can solve the problems of apf not being widely used, immunofluorescence not becoming popular in clinical practice, and hampered diagnosis of rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0053] The RA patients were recruited through Army Hospital, Department of Rheumatology, New Delhi, India. The healthy controls were requited mainly through the Guru Teg Bahadur Hospital, Delhi, India. The Army Hospital rheumatologist thoroughly reviewed the medical records of the RA patients and the characterization of the disease was based on the revised criteria of the classification of the disease by American College of Rheumatology Classification criteria. The subject sample included 107 RA affected individuals and 121 normal healthy individual, all from Indian families. The Human Ethics Committee of the Army Hospital, Department of Rheumatology, N. Delhi, Delhi, India and that of the Institute of Genoniics and Integrative Biology, Mall Road, Delhi-110007, India, approved the present study.
example 2
ELISA assay for the Detection of Autoantibodies to MBL
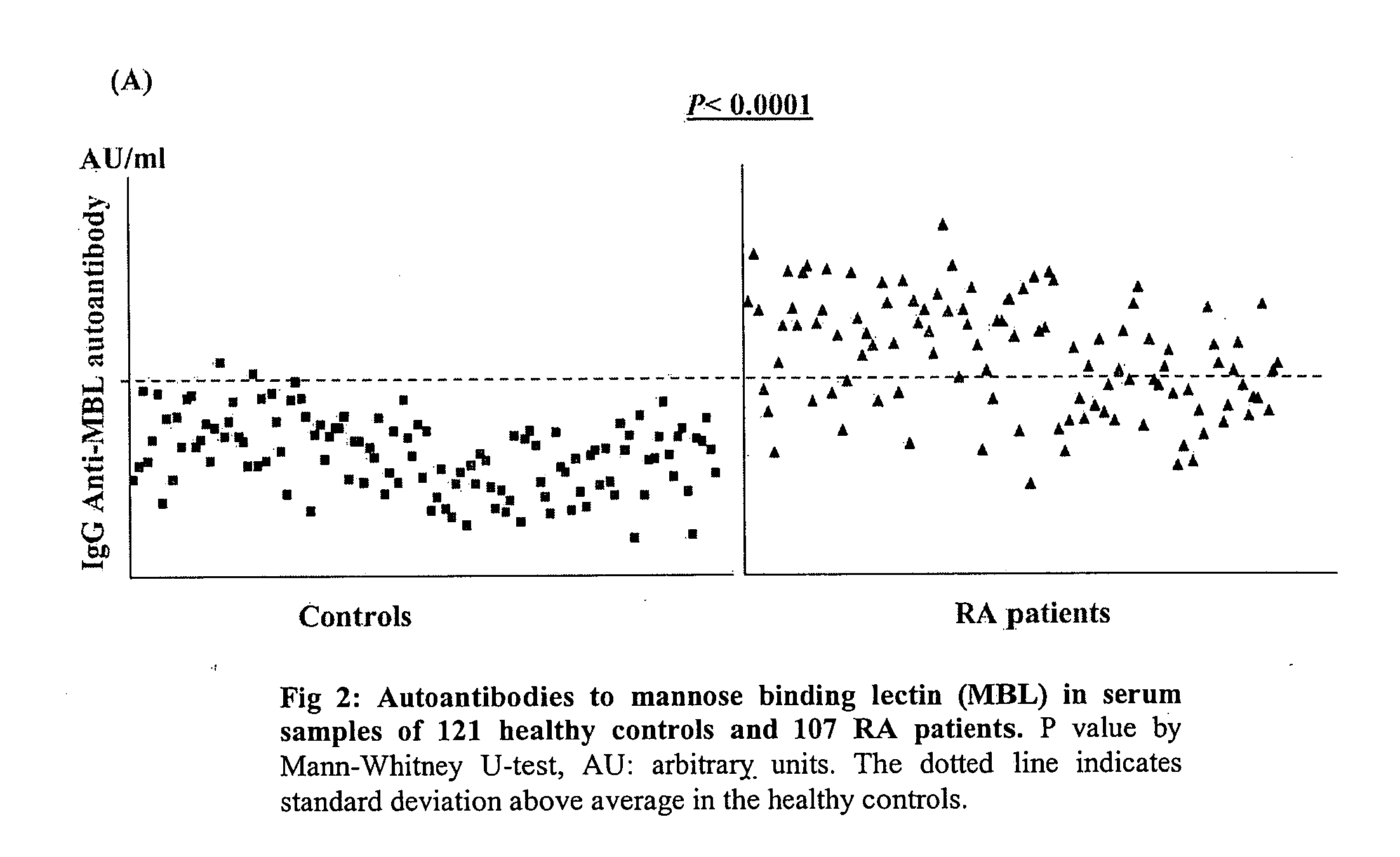
[0054] The presence of anti-MBL autoantibodies was tested for in 107 RA patients and 121 healthy controls. The ELISA tests were conducted with respect to anti-MBL antibodies, using BSA in parallel as the negative control. All the measurements were made in triplicates. The serum of the patient with a higher value of the autoantibodies to MBL was included systematically in each of the assay plates as a positive control.
[0055] The method for the identification of the autoantibodies to MBL in the sera of RA patients and the healthy controls comprises of coating an ELISA plate (Nunc) at 37° C. for two hours with 100 μl / well of serum purified MBL (U.S Biologicals) in a carbonate / bicarbonate-buffer (pH 9.6) at 0.5 μg / ml concentration. Following the steps of washing with Tris-buffered saline (TBS, pH 7.4) containing 0.05% Tween-20 (TBST), blocking of the unoccupied binding sites with 200 μl of 1% bovine serum albumin (BSA) in TBST to...
example 3
ELISA Assay for the Detection of Isotypes of Rheumatoid Factors (IgM RF and IgG RF)
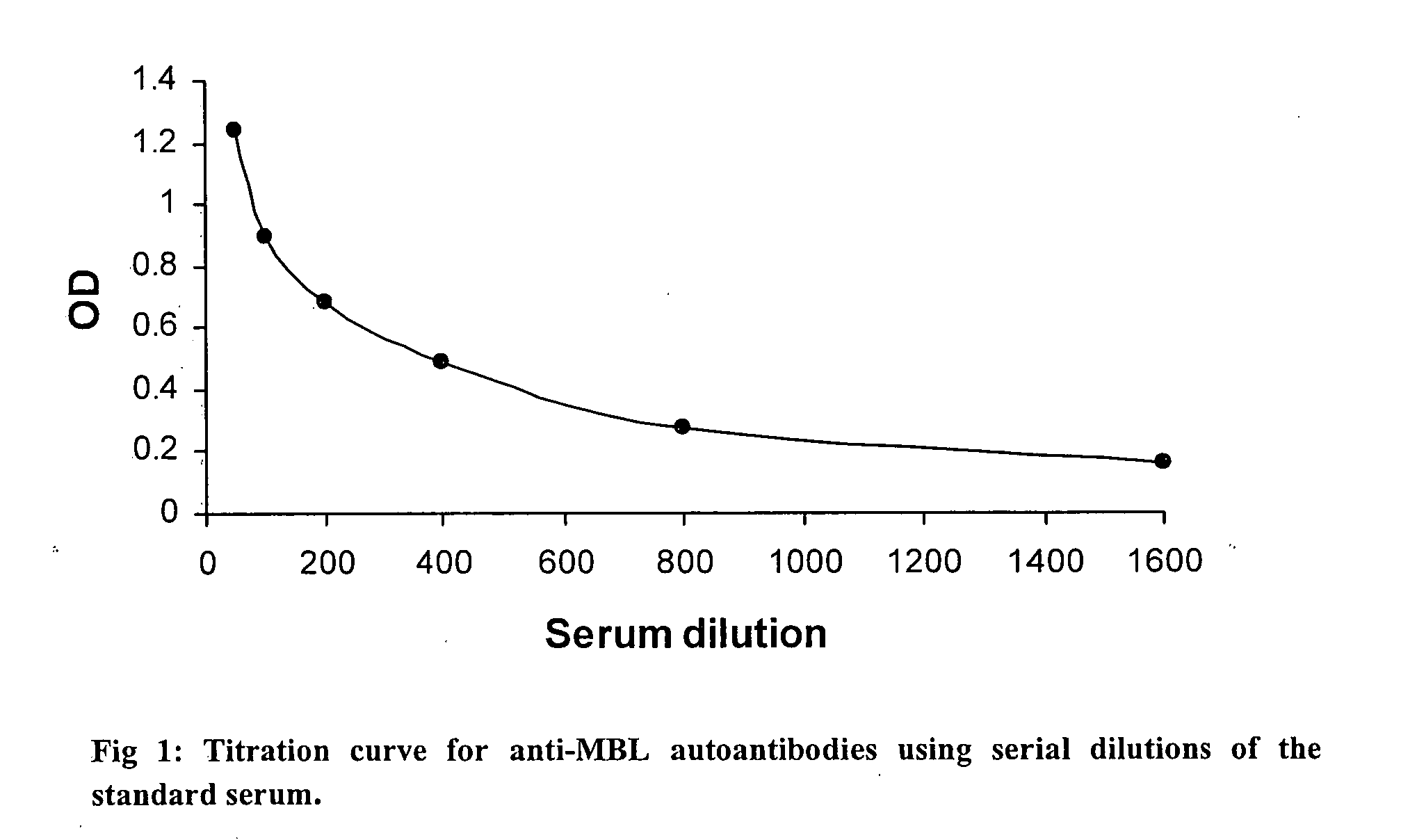
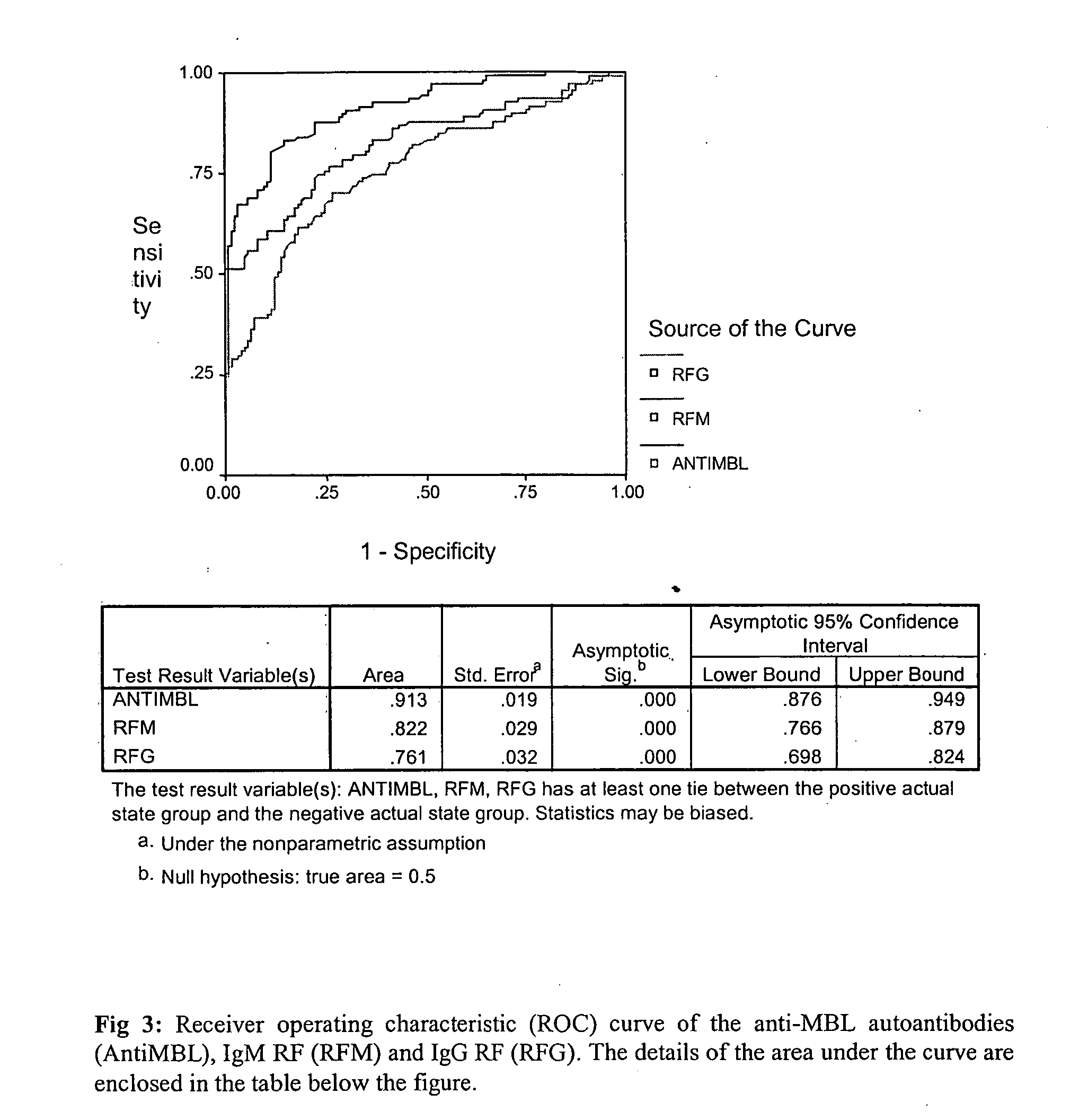
[0060] The presence of isotypes of rheumatoid factors (IgM RF and IgG RF) was measured by ELISA tests in the RA patients and the healthy controls and the standard curve was generated with each assay performed using the serial dilution of the sample used for anti-MBL autoantibody assay.
[0061] ELISA assays were developed for the measurement of rheumatoid factors of IgM and IgG isotypes in the sera of RA patients and the healthy. Flat microtitre plates (Nunc) were coated with 100 μl / well of a 10 μg / ml solution of normal rabbit IgG (Fluka) in carbonate buffer for two hours at 37° C.
[0062] Affecting the binding of rabbit IgG, the unoccupied sites were blocked using 1% BSA for 1 hour at 37° C. Subsequently the plates were washed and incubated with serum samples diluted in TBST for two hours at 37° C. The sample dilution used were 1 / 10, 1 / 50, 1 / 100 and 1 / 1000 for both IgM and IgG RF but the final dilutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| RA | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com