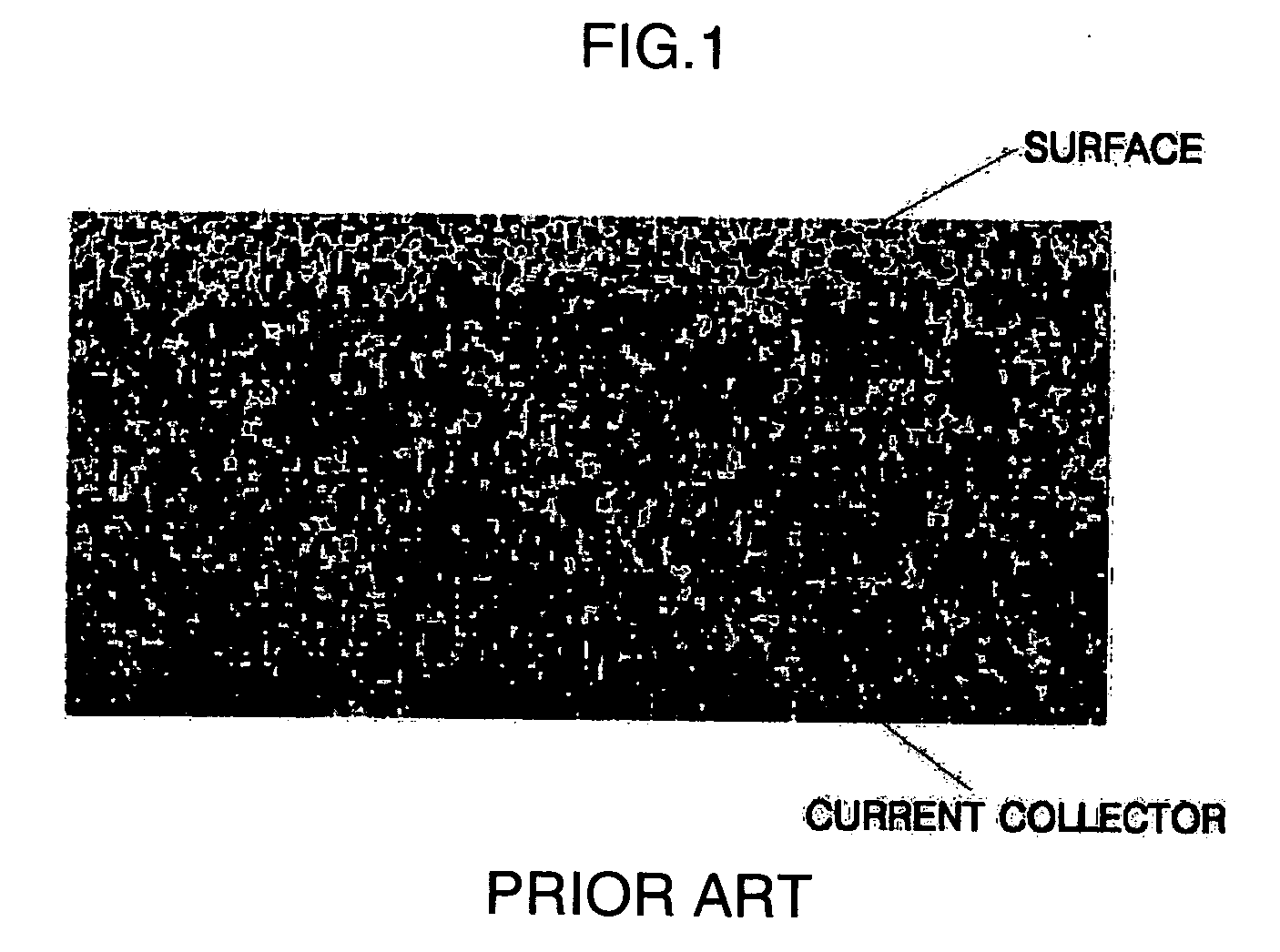
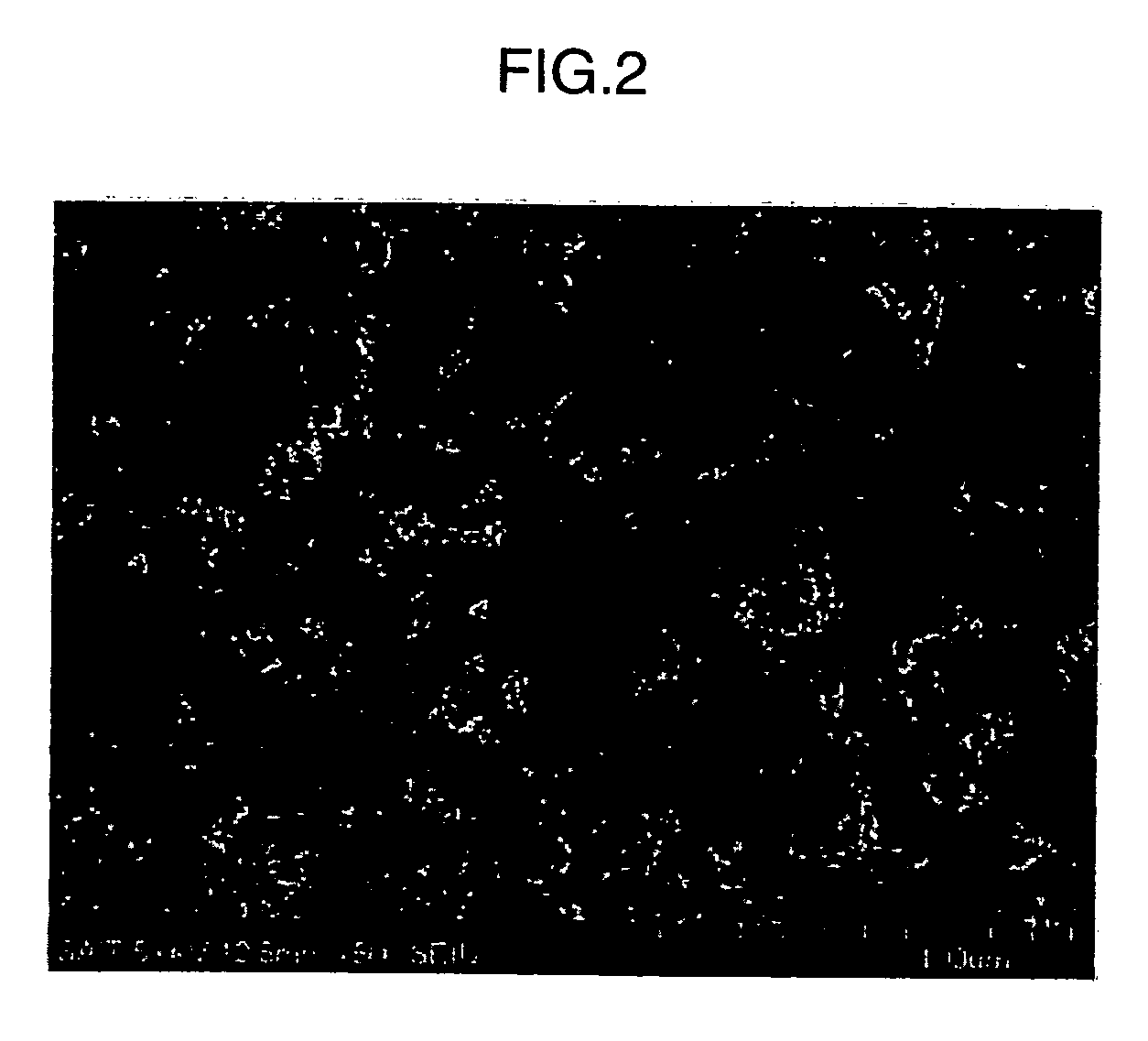
Electrode for electrochemical cell, method of manufacturing the same, and electrochemical cell includng the electrode
a technology of electrochemical cells and electrodes, applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of short circuit of electrodes, low electrical capacity, and decreased stability of metal us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0059] 150 ml of distilled water was added to a mixture of 97 g of graphite powder, 1.5 g of styrene butadiene rubber (SBR), and 1.5 g of carboxy methyl cellulose (CMC). The mixture was stirred for 30 minutes using a mechanical mixing device, thus forming a first slurry.
[0060] The first slurry was coated on a 10 μm thick Cu current collector to a thickness of about 100 μm and a loading lever of 8 mg / cm2 using a doctor blade. The first slurry was then dried.
[0061] 150 ml of distilled water was then added to a mixture of 5 g of ammonium bicarbonate, 97 g of graphite powder, 1.5 g of SBR, and 1.5 g of CMC and stirred using a mechanical mixing device for 30 minutes to produce a second slurry. The second slurry was coated on the first slurry to a loading level of about 2 mg / cm2.
[0062] The resulting coated Cu current collector was roll-pressed to a density of 1.7 mg / cm3 and dried in a vacuum at 145° C. for 3 hours, thereby forming an anode plate.
example 2
[0063] 150 ml of distilled water was added to a mixture of 97 g of graphite powder, 1.5 g of SBR, and 1.5 g of CMC and stirred for 30 minutes using a mechanical mixing device, thereby forming a slurry.
[0064] The slurry was coated on a 10 μm thick Cu current collector to a loading level of 10 mg / cm2 using a doctor blade. The slurry was then dried.
[0065] The coated Cu current collector was further coated by spraying it with ammonium bicarbonate dissolved in ethanol. The ammonium bicarbonate was coated to a loading level of about 0.1 mg / cm2.
[0066] The coated Cu current collector was roll-pressed to a density of 1.7 g / cm3 and then dried in a vacuum at 145° C. for 3 hours, thereby forming an anode plate.
example 3
[0067] An anode plate was manufactured as in Example 1 except that ammonium oxalate was used instead of ammonium bicarbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com