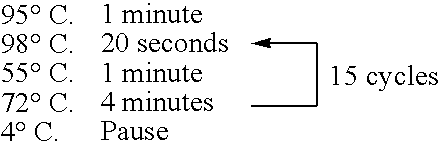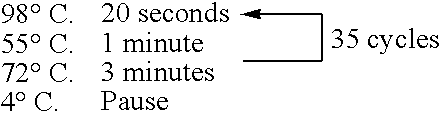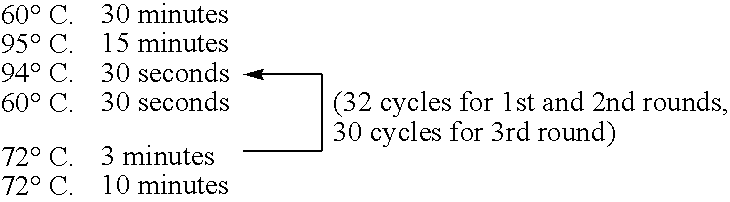Protein forming complex with c-fos protein, nucleic acid encoding the same and method of using the same
a technology of protein forming complex and c-fos protein, which is applied in the field of protein, can solve the problems of enormous time and labor required to achieve 1:1 molecule analysis technique and the use of two hybrid methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0367] Cotranslation selection / screening of IVV was carried out by using the c-Fos protein as a bait and a mouse brain cDNA library as a prey (FIG. 2), and as a result, genes or nucleic acid sequences encoding novel proteins that can form a complex with the c-Fos protein were obtained.
[0368] The preparation method of the bait, c-Fos protein, was as follows. A DNA template was prepared from a pCMV-FosCBPzz vector (SEQ ID NO: 168) by PCR (primers 5′ SP6(O29)T7-FosCBPzz (SEQ ID NO: 169) and 3′ FosCBPzz (SEQ ID NO: 170), and PCR program CYCB1 (refer to Table 1)) using TaKaRa Ex Taq (Takara Shuzo). The DNA template was transcribed (37° C., 2 hours) by using RiboMAX™ Large Scale RNA Production Systems (Promega) to prepare a mRNA template of the bait c-Fos protein. A bait DNA made to coexist was prepared by PCR (primers 5′ DNA (SEQ ID NO: 172) and 3′ DNA (SEQ ID NO: 173)) using DNA-Fos / Jun (SEQ ID NO: 171) containing the Fos / Jun binding sequence as a template according to the PCR program ...
example 2
[0374] A prey IVV library was prepared from the bait c-Fos and a mouse brain cDNA library in the same manner as that used in Example 1, and the screening / selection procedure (FIG. 2) was also performed in the same manner as that used in Example 1. However, in this example, the first stage selection using IgG beads in the two-stage screening was repeated 3 times, and the two-stage selection was performed only for the 4th time to obtain the proteins of SEQ ID NOS: 47 to 56 (amino acid sequences of Fip-cx.1), SEQ ID NOS: 57 to 76 (amino acid sequences of Fip-cx.2), SEQ ID NOS: 77 to 81 (amino acid sequences of Optin), SEQ ID NOS: 82 to 84 (amino acid sequences of Snap19), SEQ ID NOS: 85 and 86 (amino acid sequences of C130020M04Rik), SEQ ID NOS: 87 to 89 (amino acid sequences of FLJ32000), SEQ ID NOS: 90 and 91 (amino acid sequences of Rit2), SEQ ID NOS: 92 and 93 (amino acid sequences of cytocrome b), SEQ ID NOS: 94 and 95 (amino acid sequences of Apoe), SEQ ID NOS: 96 and 97 (amino a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dA | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com