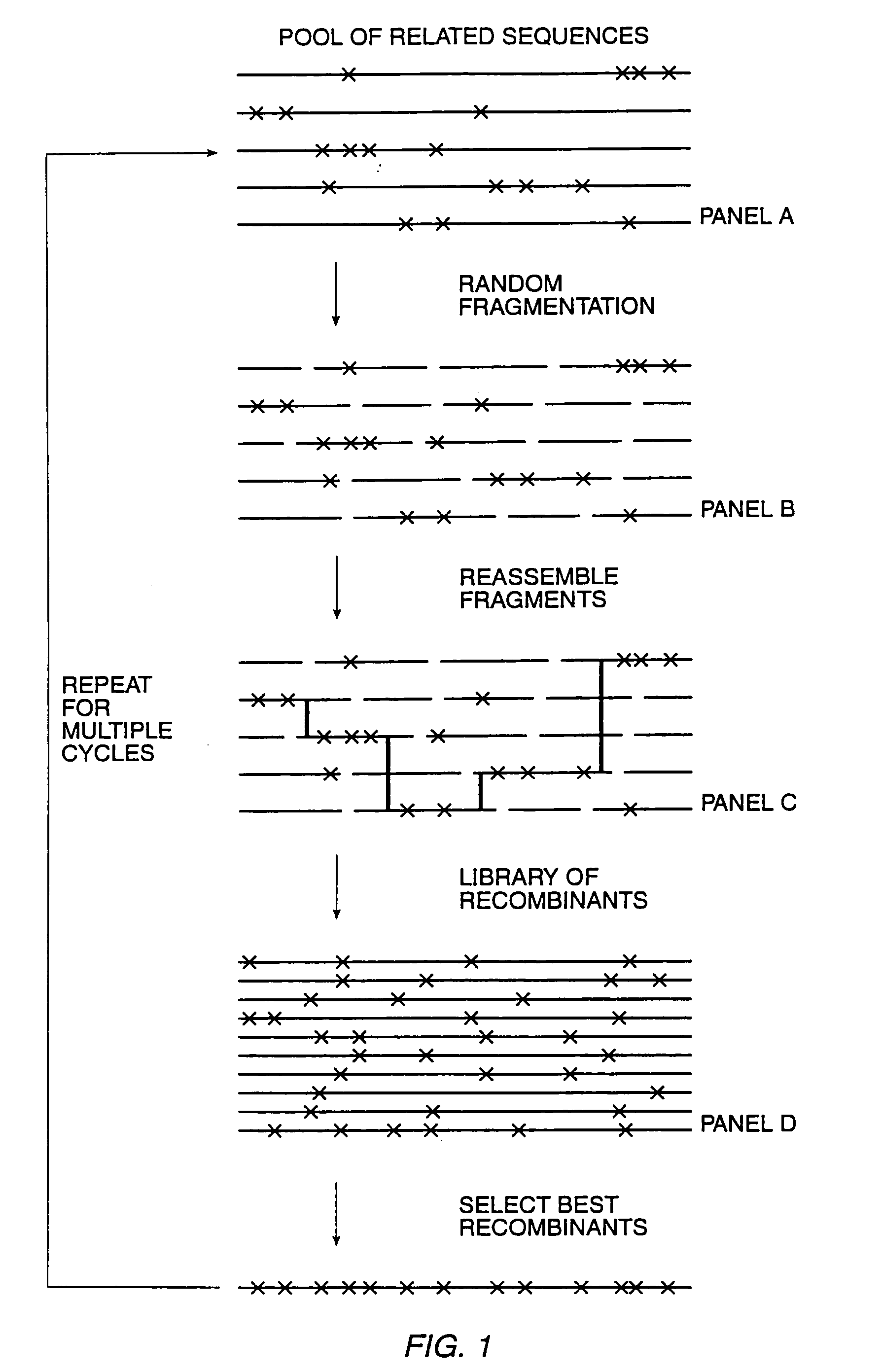
Methods and compositions for cellular and metabolic engineering
a metabolic engineering and cellular technology, applied in the field of methods and compositions for cellular and metabolic engineering, can solve the problems of unsuitable intracellular location of proteins, unsuitable folding, improper modification, etc., and achieve the effect of improving the ability to catalyz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
I. Alteration of Enzyme Activity and Specificity.
[0208] In this example, recursive sequence recombination techniques of the instant invention were used to expand the range of substrates efficiently hydrolyzed by E. coli β-galactosidase. The goal was to evolve wild type E. coli β-galactosidase into a fucosidase. The enzyme showed very weak activity with both ρ-nitrophenyl-β-D-fucopyranoside and o-nitrophenyl-β-D-fucopyranoside (estimated respectively as 80- and 160-fold less efficient than for ρ-nitrophenyl-β-D-galactopyranoside).
[0209] To increase the activity of E. coli β-galactosidase against these fucopyranoside derivatives, a lacZ gene (a 3.8 kb Hind III-BamHI fragment from plasmid pCH110, Pharmacia) encoding E. coli β-galactosidase was subcloned into plasmid p18SFI-BLA-SFI (Stemmer, Nature, 370:389-391 (1994)). The resulting plasmid, p18-lacZ, was used for recursive sequence recombination and mutant screening.
[0210] Purified plasmid p18-lacZ (4-5 μg) was used directly for D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com