Closed loop impedance-based cardiac resynchronization therapy systems, devices, and methods
a closed loop and impedance-based technology, applied in the field of closed loop resynchronization therapy systems, devices, and methods, can solve the problems of reduced blood circulation, poor spatial coordination of patient's heart contraction, and both irregular rhythms of patients' heart contractions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
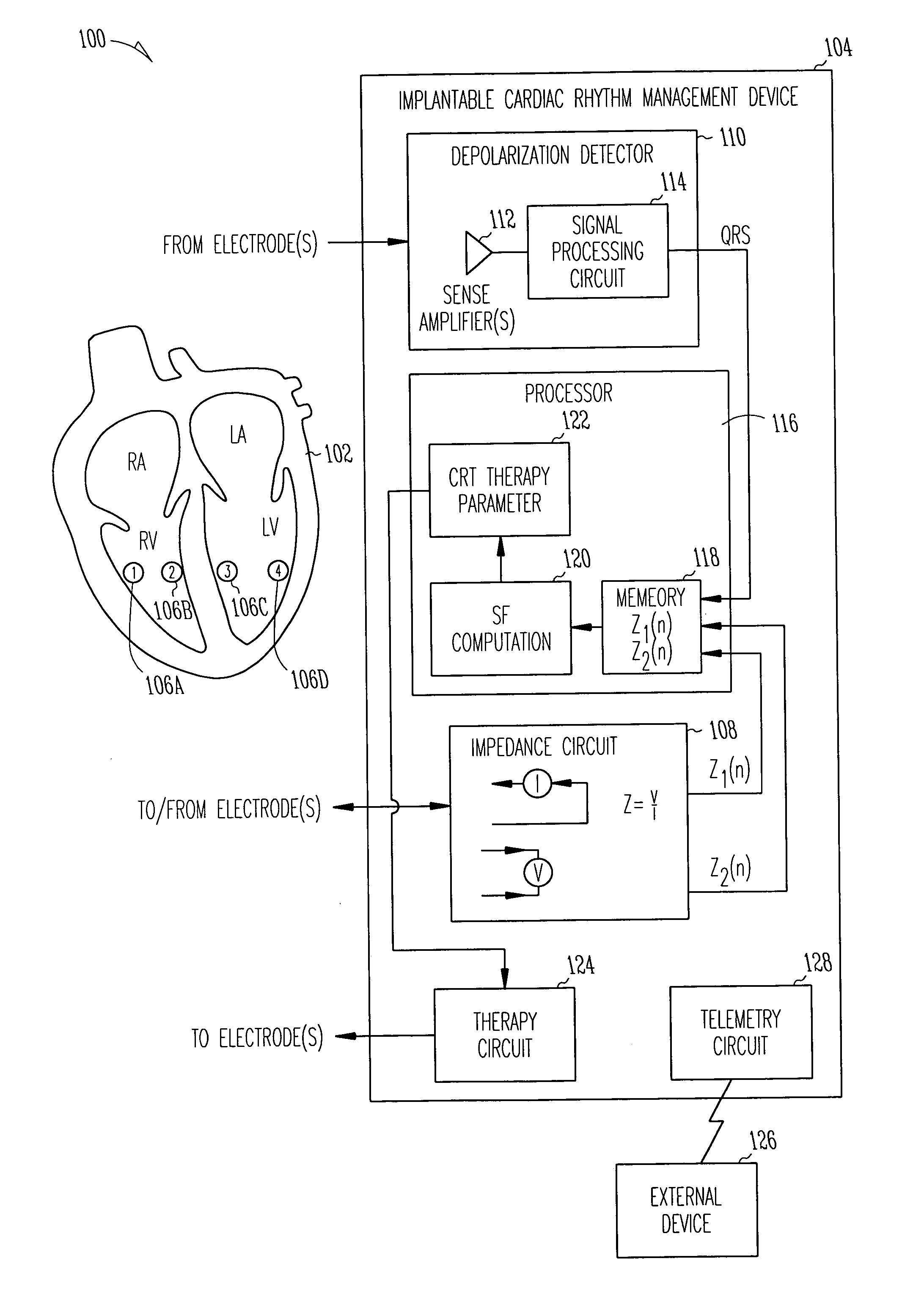
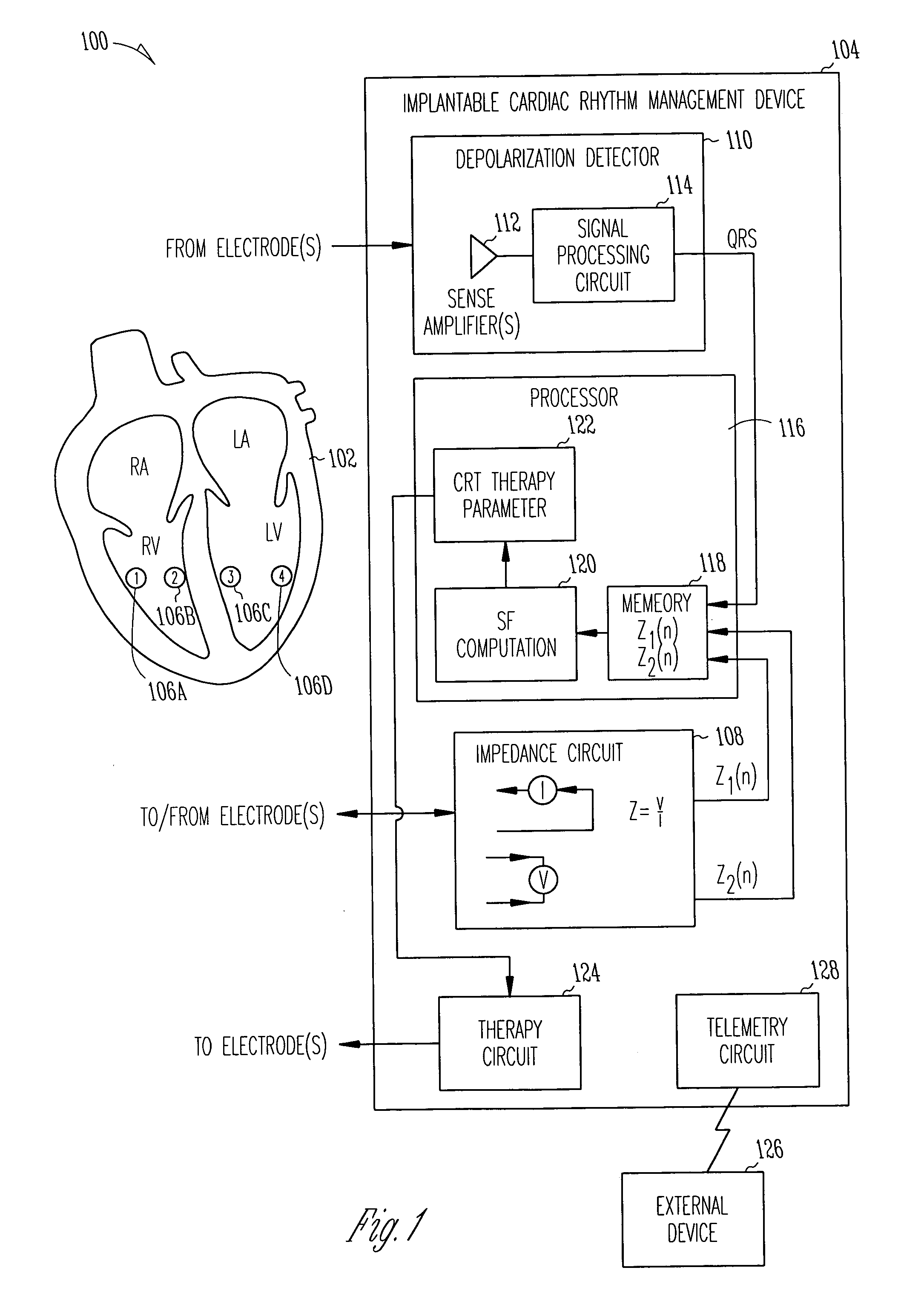
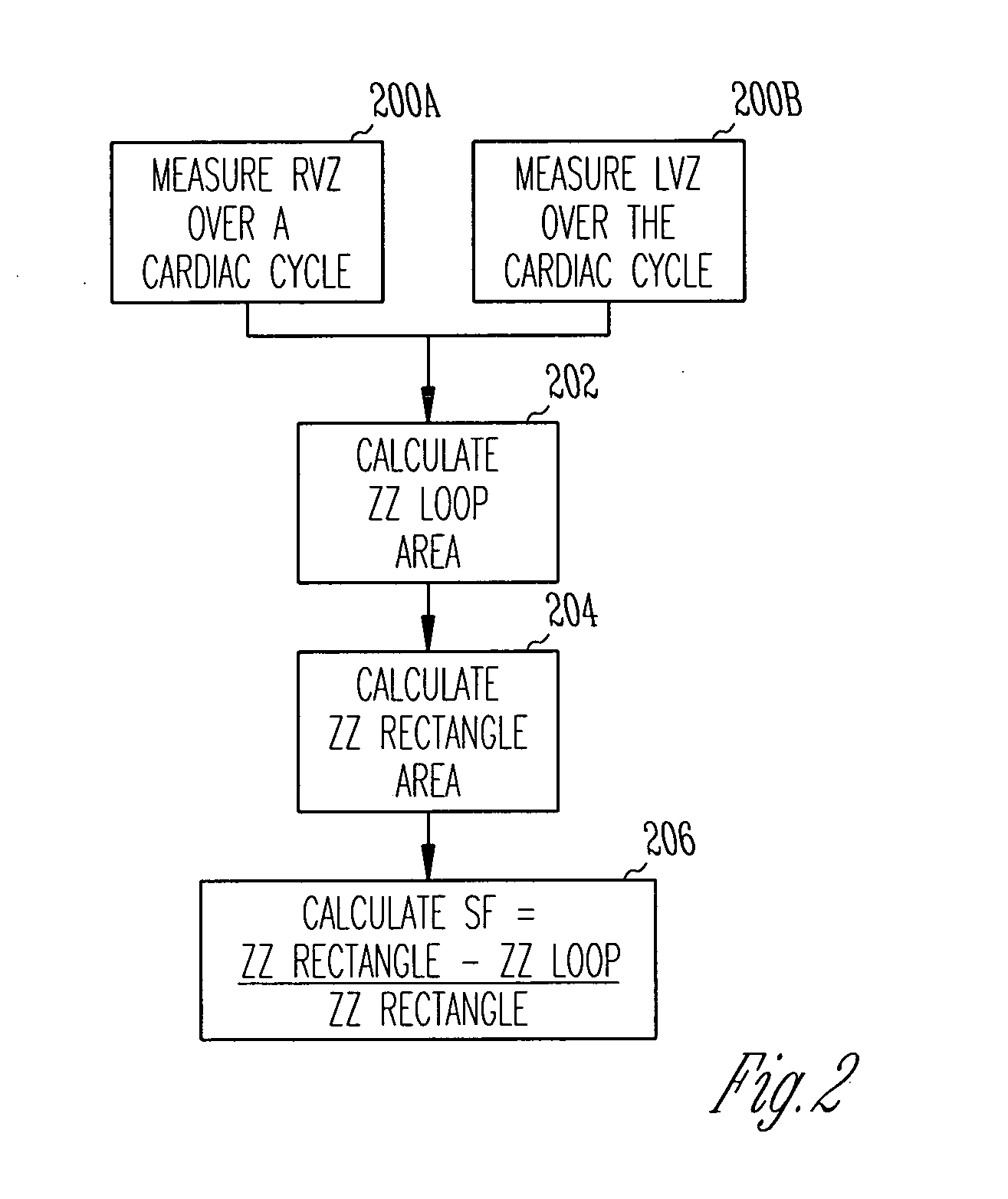
[0040]FIG. 1 is a schematic diagram illustrating generally one example of portions of a system 100 and portions of an environment with which it is used, including a heart 102. In this example, the system 100 includes an implantable cardiac function management device 104. In one example, the device 104 is coupled to the heart 102 using one or more intravascular or other leadwires. The leadwires provide electrodes 106 in association with the heart 102. FIG. 1 illustrates an example that includes a first electrode 106A that is located at or near a right ventricular freewall, a second electrode 106B that is located at or near a right ventricular septum, a third electrode 106C that is located at or near a left ventricular septum, and a fourth electrode 106D that is located at or near a left ventricular freewall. This particular electrode configuration of FIG. 1 is useful for providing conceptual clarity, however, other possibly more practical electrode configurations will be discussed fu...
example 2
[0057]FIG. 6 is a schematic diagram illustrating generally one example of portions of a system 600 and portions of an environment with which it is used, including a heart 602. In this example, the system 600 includes an implantable cardiac function management device 604. In one example, the device 604 is coupled to the heart 602 using one or more intravascular or other leads. The leads provide electrodes 606 in association with the heart 602. FIG. 6 illustrates an example that includes a first electrode 606A that is located at or near an midportion of a right ventricular freewall, a second electrode 606B that is located in association with a left ventricular freewall, such as by being introduced on an intravascular lead that is inserted into coronary sinus 607 toward a coronary sinus vein. A third electrode 606C is located on a hermetically-sealed housing (“can”) of the implantable device 604 (or, alternatively, on an insulating “header” extending from the housing of the implantable...
example 3
[0069]FIG. 10 is a schematic diagram illustrating generally one example of portions of a system 1000 and portions of an environment with which it is used, including a heart 1002. In this example, the system 1000 includes an implantable cardiac function management device 1004. In one example, the device 1004 is coupled to the heart 1002 using one or more intravascular or other leads. The leads provide electrodes 1006 in association with the heart 1002. FIG. 10 illustrates an example that includes a first electrode 1006A that is located at or near a middle or apical portion of a right ventricular septum, a second electrode 1006B that is located in association with a left ventricular freewall, such as by being introduced on an intravascular lead that is inserted into coronary sinus 1007 toward a lateral or posterior coronary sinus vein.
[0070] In FIG. 10, the device 1004 includes an impedance circuit 1008 for measuring a left ventricular impedance between the first electrode 1006A and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com