Method for treating amyloid disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
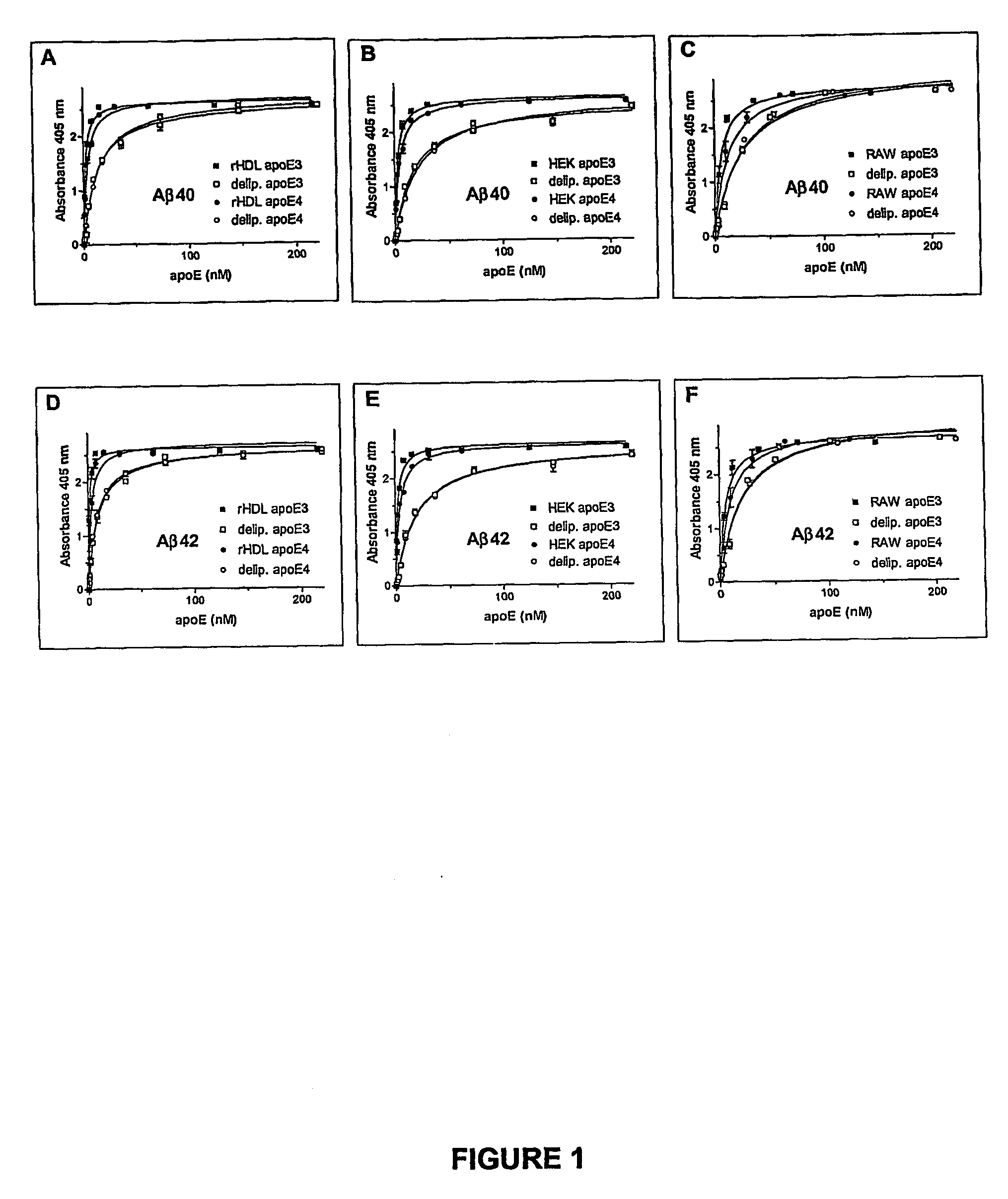
Binding of ApoE Preparations to Aβ Peptide
[0095] In the present Example, the binding of apoE, derived from various sources and in various forms, to Aβ1-40 and Aβ1-42 peptides is evaluated.
Materials and Methods
[0096] Synthetic Peptides and Proteins. The following peptides, DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV (SEQ ID NO:1), and DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO:2) corresponding to Aβ40 and Aβ42, and identical to residues 672-711 and 672-713 of Aβ-precursor protein 770, respectively, were synthesized at the W. M. Keck Facility at Yale University (New Haven, Conn., U.S.A.) using N-t-butyloxycarbonyl chemistry and purified by HPLC. Aliquots of the final products were lyophilized and stored at −20° C. until use. For preparation of aggregated peptides, 50 μg of either Aβ40 or Aβ42 were dissolved in 100 μl of PBS (0.02 M phosphate buffer, pH 7.4, containing 0.15 M NaCl) and incubated at 37° C. for 72 h. ApoE3 and apoE4 produced in Sf9 insect cells by the baculovi...
example 2
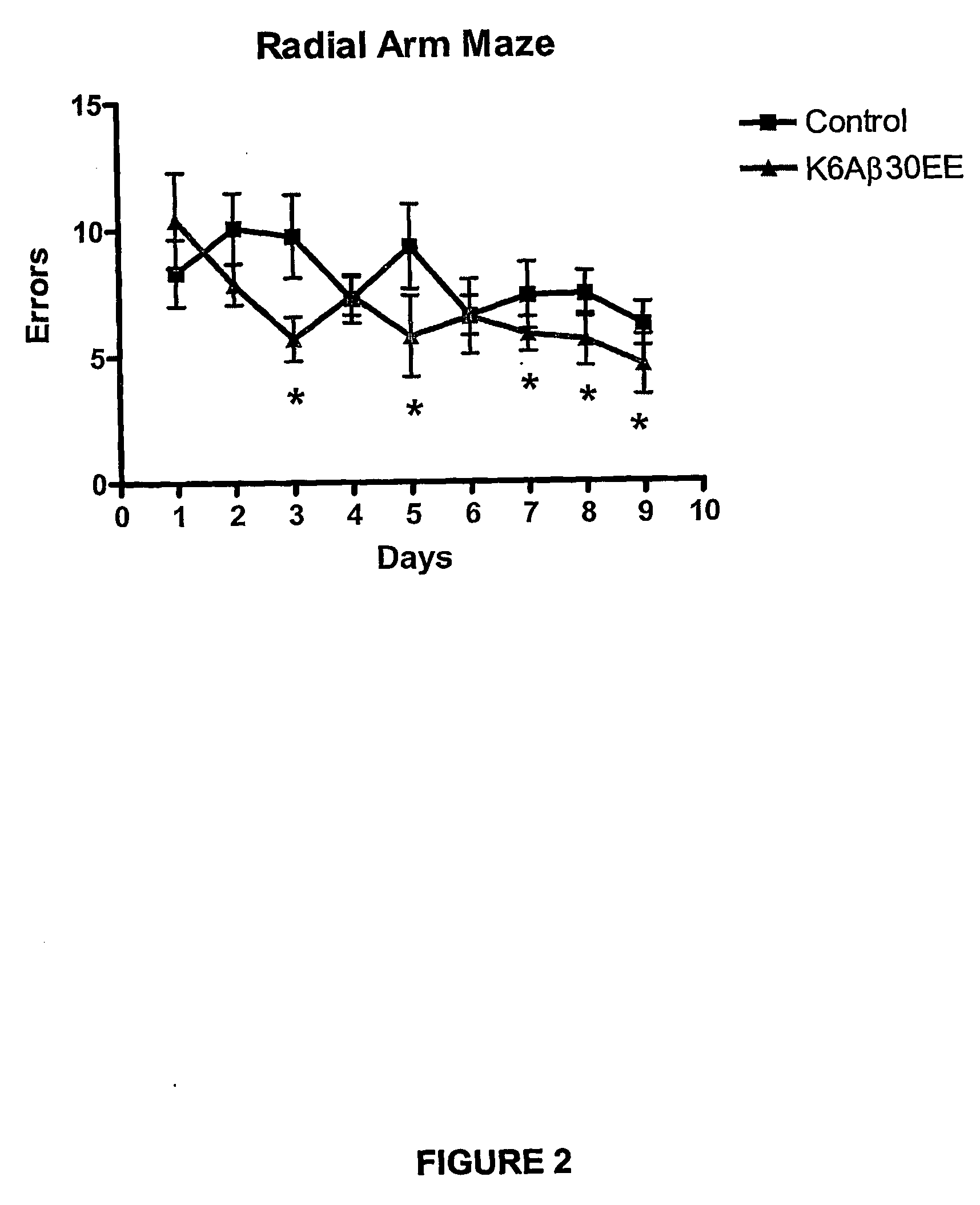
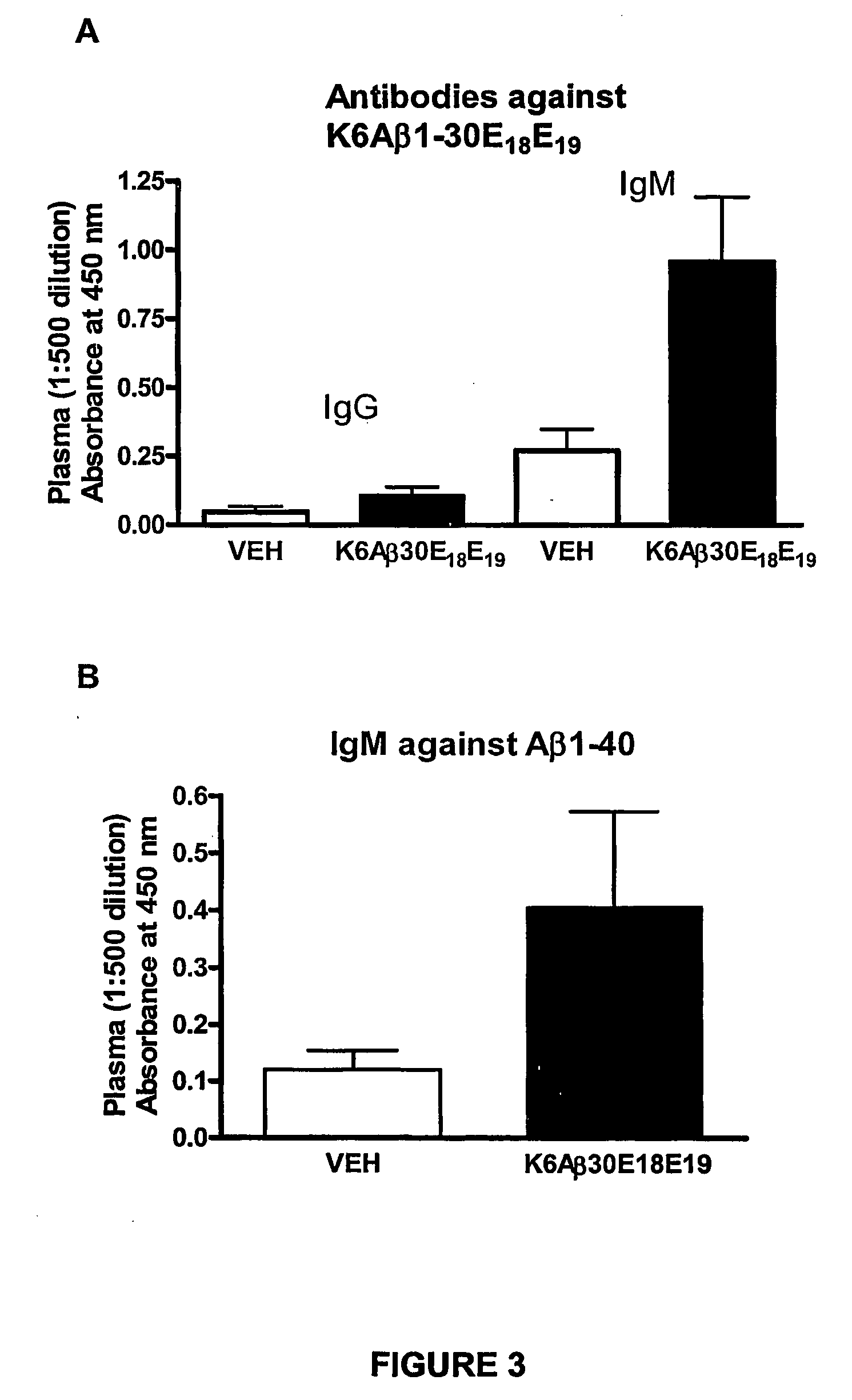
Elicitation of Anti-Aβ-Antibodies Removing Aβ from Blood
[0109] This Example describes immunization of animals with synthetic Aβ-derivatives, with subsequent analysis of Aβ content in brain and blood after in vivo formation of anti-Aβ-antibodies. According to the invention, similar results could be obtained by administering externally produced antibodies against Aβ.
[0110] Briefly, mice received their first immunization with K6Aβ1-30-NH2(E18E19) at 10.5-13 months of age (peptide: n=23; vehicle: n=24). The animals were bled prior to vaccination, 3 months following the first injection and at the time of sacrifice at 18 to 21 months. The mice were tested in the radial arm maze, subsequently perfused and their brains processed as described (Sigurdsson et al., Am. J. Pathol. 2001; 159:439-4471) (peptide: n=18; vehicle: n=18).
Materials and Methods
[0111] Peptide. K6Aβ1-30-NH2(E18E19) was synthesized at the Keck Foundation (Yale University, New Haven, Conn.). It consists of the first 30 a...
example 3
Apolipoprotein E3 (ApoE3) Treatment of Patients with Alzheimer's Disease
[0122] The present example evaluates the pharmacokinetics and treatment effects of administered ApoE3 versus placebo adjunctive treatment in patients with Alzheimer's disease. Either free, recombinantly produced apoE protein, or apoE protein incorporated into HDL particles can be used in this method. In some cases, apoE incorporated into HDL particles may be preferable, as this is the form apoE is generally found in vivo.
Materials and Methods
[0123] The study is a randomized, double blind, placebo controlled parallel group study, and includes patients meeting the following inclusion criteria. Participants must either have genetic mutations that will result in Alzheimer's disease or be diagnosed with the disease, and may be male or female. Parents or guardians will give informed consent for those under the age of 18 years. Exclusion criteria include females who are pregnant or nursing; life-threatening infectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body weight | aaaaa | aaaaa |
| Body weight | aaaaa | aaaaa |
| Absorption cross section | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com