Cell or drug encapsulation device having a wet seal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
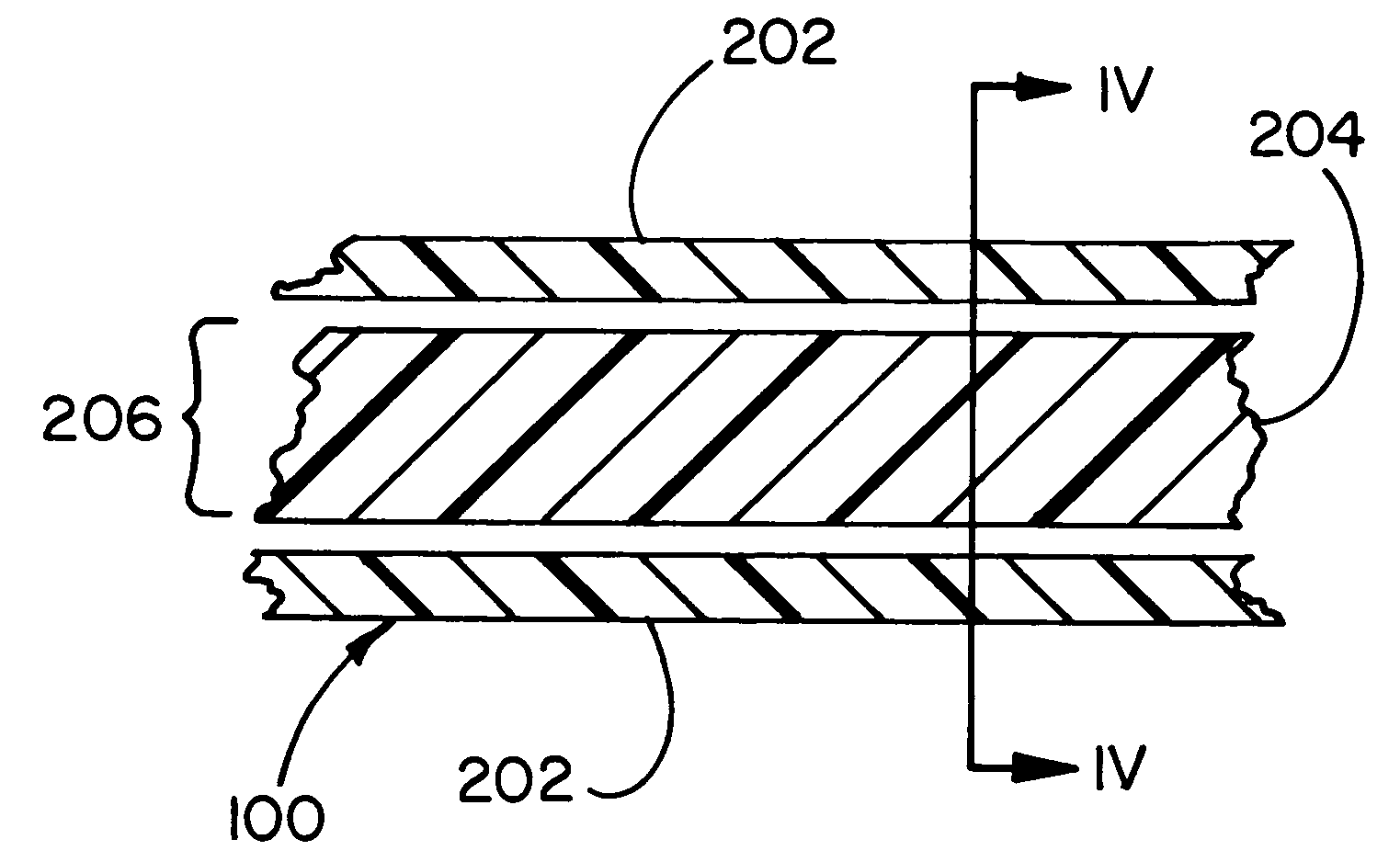
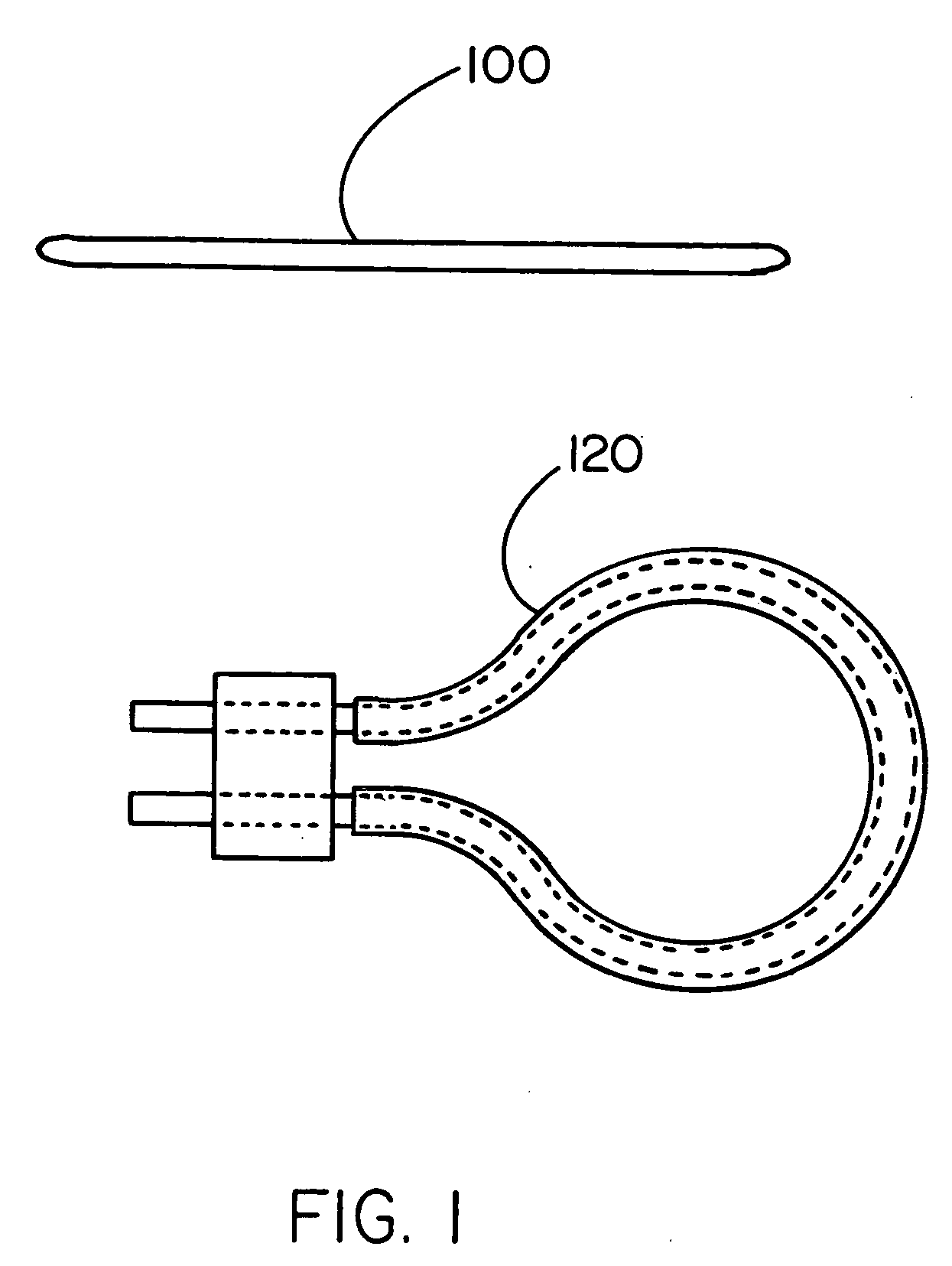
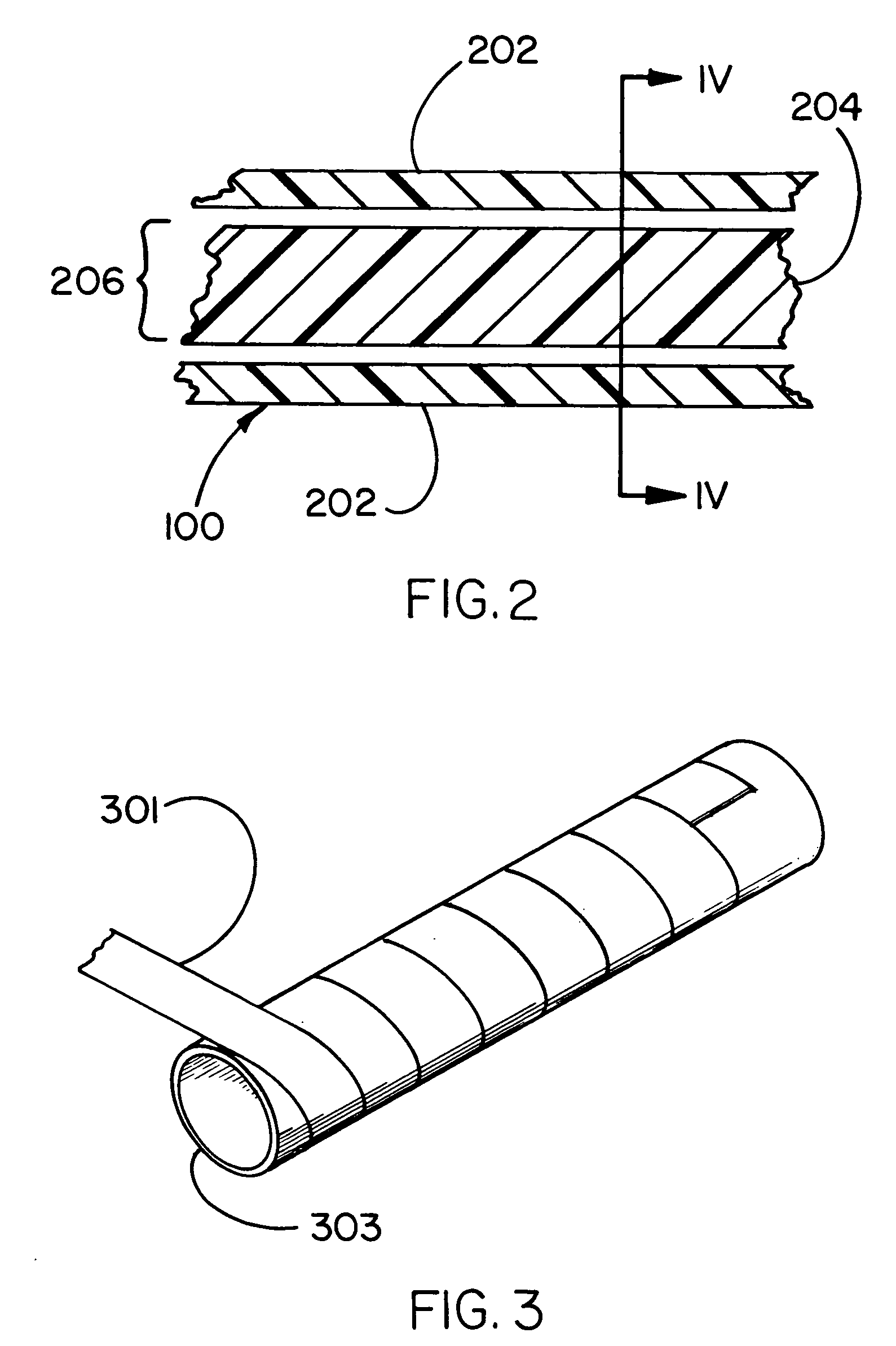
[0045] The invention provides a containment device, methods of making the device and methods of loading and sealing the device. The containment device is particularly suited for use as a medical device, such as a cell encapsulation device, a drug delivery device, or a gene therapy device. The containment device may be inserted into a previously implanted containment apparatus residing within a recipient, such as an animal or human, or it may be implanted directly in a recipient. The device includes a permeable membrane, which partially defines an enclosed space of the device, and a closure region of the device. Materials (e.g., cells, or drugs) are loaded into the device from a loading device through the closure region into a containment region, after which the closure region is treated to form a closure. The closure is typically created by heating a thermoplastic polymer associated with the permeable membrane in the presence of a liquid that wets at least a portion of the membrane ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Permeability | aaaaa | aaaaa |
| Heat | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com