Methods of preparation of an olefin oligomerization catalyst
a technology of oligomerization catalyst and olefin, which is applied in the field of preparation of catalysts, can solve the problems of low product yield and lack of selectivity to a desired produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0180] Catalyst 1-8
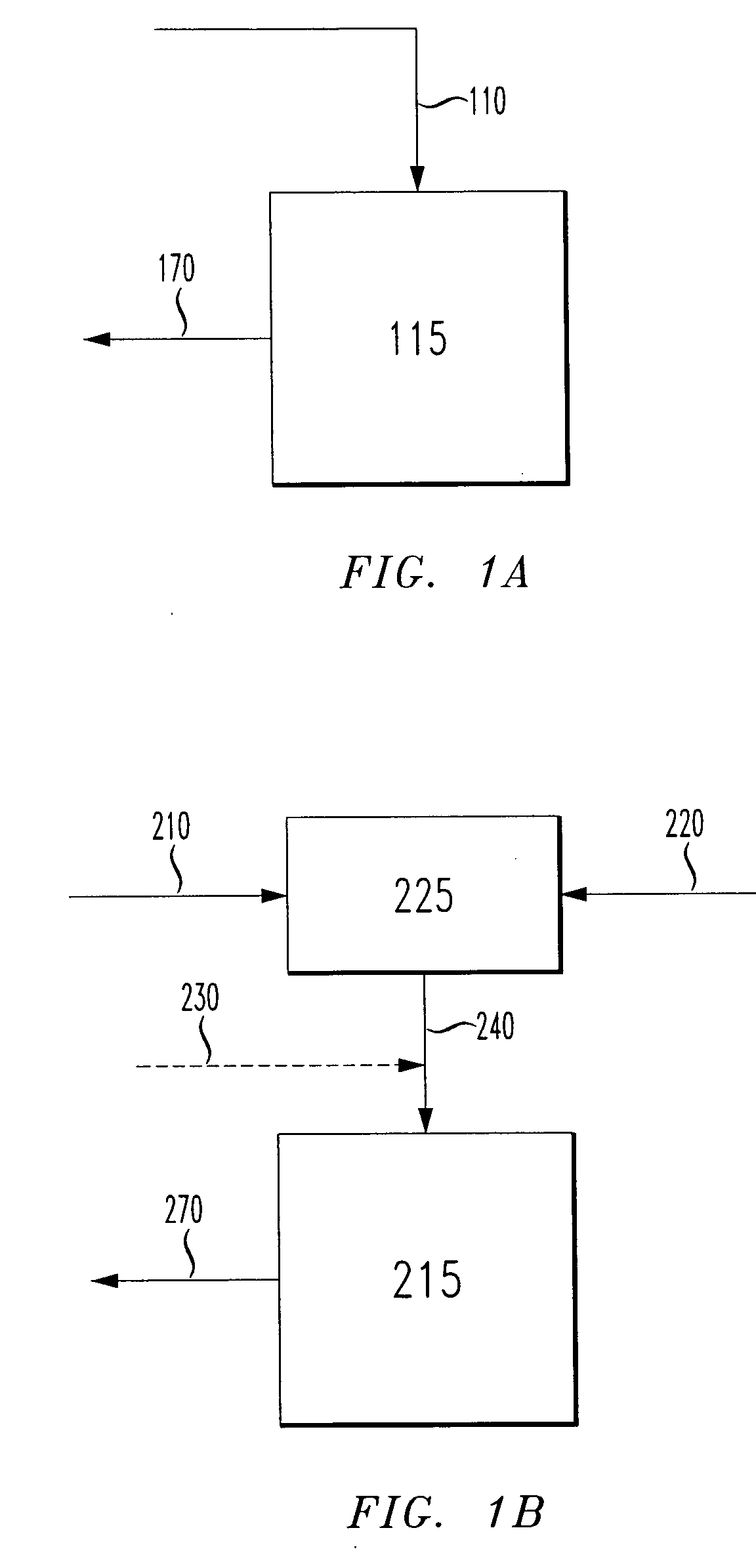
[0181] Catalyst was prepared by adding 14.1 lbs of dry, nitrogen-purged toluene to a 5 gallon reactor. To the toluene was added 630.9 g chromium(III) 2-ethylhexanoate dissolved in 750 mL toluene followed by a 300 mL toluene rinse. 2,5-Dimethylpyrrole (388.9 mL) was added to the chromium solution in the reactor. The reactor was purged with nitrogen and brought to a temperature of 25° C. A mixture of 1,600 g neat triethylaluminum (TEA) and 1,229 g neat diethylaluminum chloride (DEAC) was then added to the reactor followed by 0.2 lbs of toluene rinse. The temperature increased 18° C. and was returned to 25° C. with cooling. The contents of the reactor stood overnight and were then filtered, using a filter comprising a combination of a metal screen, filter paper, glass wool, diatomaceous earth, and another layer of glass wool. Additional catalysts were prepared in which the temperature and chromium concentration of the catalyst preparations were varied. The catalysts ...
example 2
[0183] Catalyst 9-10
[0184] An ethylbenzene solution containing 2.3 g chromium(III) 2-ethylhexanoate and 8.13 g ethylbenzene was prepared. A separate solution containing 6.05 g neat triethylaluminum (TEA), 4.63 g neat diethylaluminum chloride (DEAC), 1.37 g 2,5-dimethylpyrrole and 22.6 g ethylbenzene was also prepared. These two solutions were added to 30.98 g of active catalyst over a 40 minute period such that the addition time for both solutions started and ended at the same time. The catalyst was tested in a 1 L continuous reactor and the average results of two test runs are shown in Table 2 as Catalyst 10. The average of two test runs of a standard catalyst preparation is shown in Table 2 as Catalyst 9.
TABLE 2Select-TotalivityPurityProductivityRx PolymerPolymerCatalyst(1-C6=)(1-C6=)(g 1-C6= / g Cr)(Lb / Hr 100 MM / yr Plant) 989.3%98.8%82,5750.0013.331089.1%98.7%82,9890.007.18
[0185] The examples show that an acceptable catalyst can be prepared. The examples further indicate that a ...
example 3
[0186] Catalyst 11
[0187] A solution was prepared by mixing 12.10 g neat triethylaluminum (TEA), 9.38 g neat diethylaluminum chloride (DEAC) and 20.02 g ethylbenzene. Two aliquots were added to this solution. The first contained 2.3 g chromium(III) 2-ethylhexanoate, 1.14 g ethylbenzene and 2.74 g 2,5-dimethylpyrrole. The second contained 2.3 g chromium(III) 2-ethylhexanoate and 1.14 g ethylbenzene. Ethylbenzene was added to obtain a total volume of 100 mL. The catalyst prepared by this method was tested in a 1 L continuous reactor. The average results of three test runs are shown in Table 3.
TABLE 3SelectivityPurityCatalyst ProductivityCatalyst(1-C6=)(1-C6= / C6)(g 1-C6= / g Cr)1191.2%99.2%80,759
[0188] The example shows high selectivity (91.2%), high purity (99.2%), and good catalyst productivity (80,759 g 1−C6= / g Cr) for the catalyst preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com