Apoptotic entities for use in treatment of neurodegenerative and other neurological disorders
a neurodegenerative and other neurological disorder technology, applied in the field of biochemical and biological compositions, can solve the problems of harmful inflammatory consequences, sensitive enough to detect apoptosis,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0042] Experiments to demonstrate the invention were conducted on laboratory mice, under approved conditions for conducting such experiments.
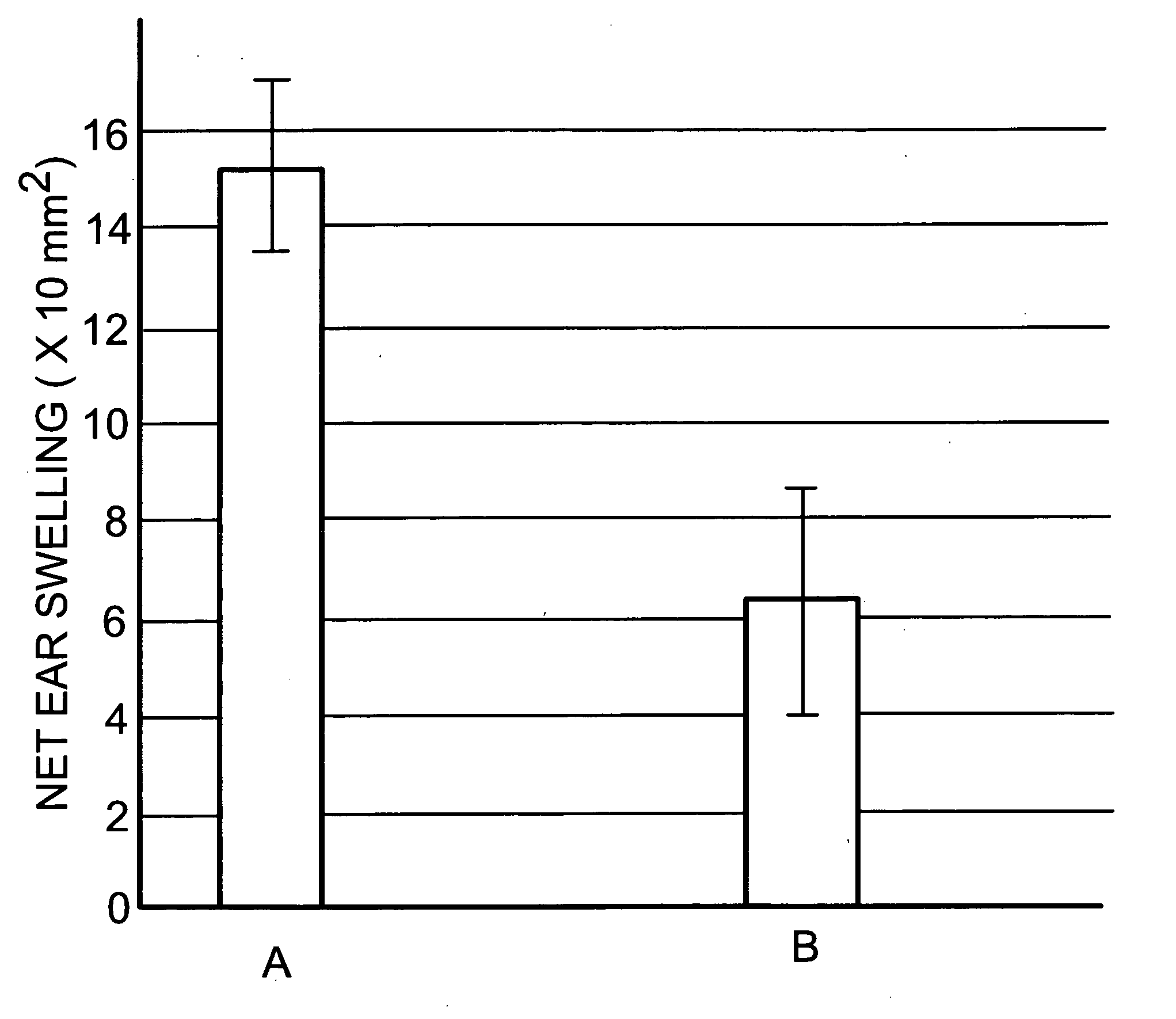
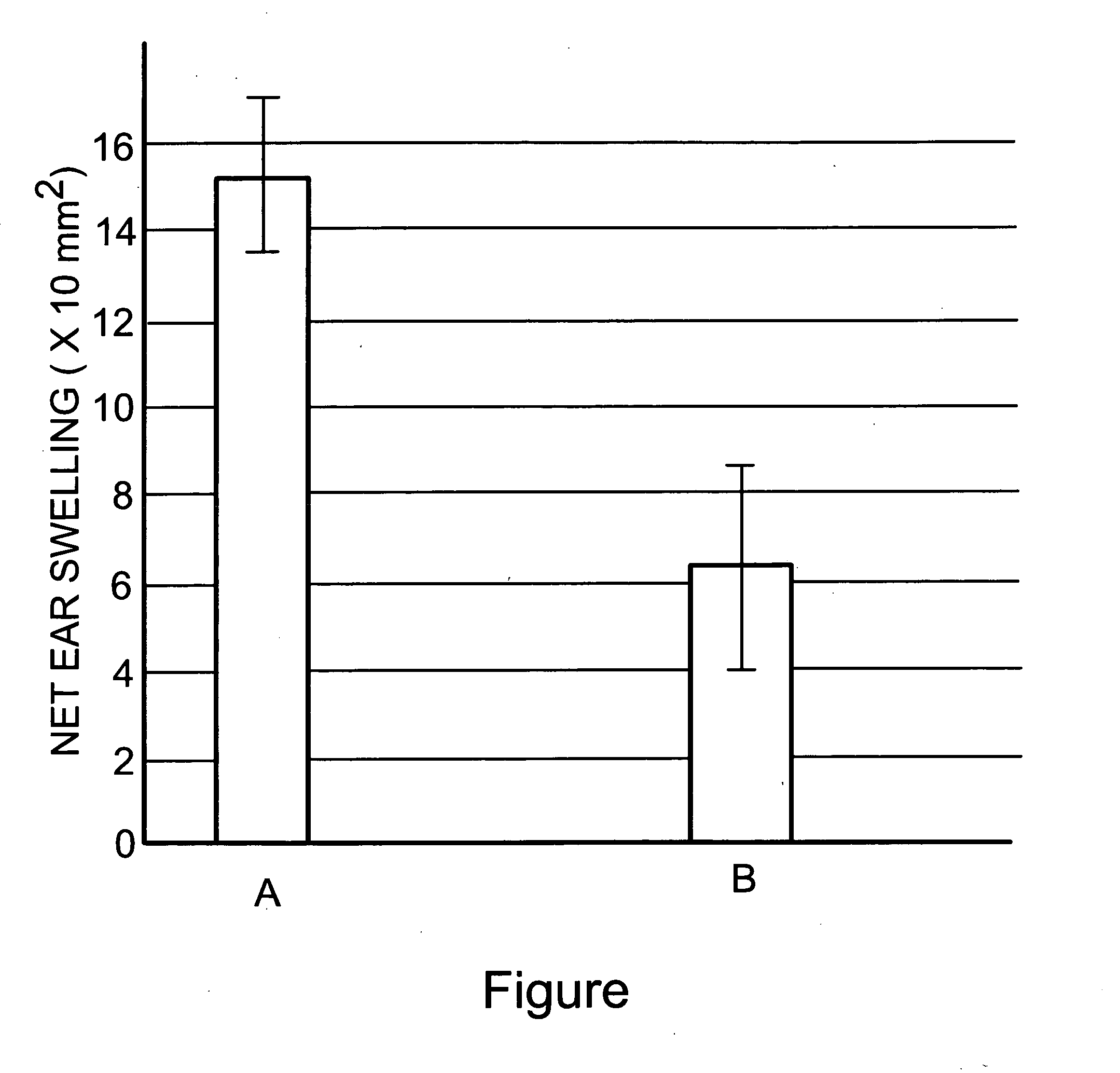
[0043] The effectiveness of the treatment according to a preferred embodiment of the present invention, on contact hypersensitivity (CHS), an example of a Th-1-cell inflammatory disorder which is known to be mediated by inflammatory cytokines, was assessed on laboratory mice, according to approved animal experimentation procedures, using the method described by Kondo et. al., “Lymphocyte function associated antigen-1 (LFA-1) is required for maximum elicitation of allergic contact dematitis” Br. J. Dermatol. 131:354-359 (1994), with minor variations. The disclosure thereof is incorporated herein by reference. Briefly, to induce C H S, the abdominal skin of each mouse was shaved and painted with dinitrodifluorobenzene DNFB, the sensitizing chemical, using 25 μl of 0.5% DNFB in 4:1 acetone:olive oil solution. This sensitization was applied to two...
example 2
[0049] The above test procedure was repeated on similar groups of animals, a control group and a test group, but using a suspension of apoptotic cells and bodies on the test group which comprised about 60% apoptotic cells and bodies, balance viable cells and a minor amount (not more than 20%) of necrotic cells. Essentially similar results were obtained.
[0050] The effectiveness of the processes and compositions of the present invention in preventing and alleviating inflammation due to CHS indicates that administration of apoptotic cells and bodies as described up-regulates the in vivo generation of anti-inflammatory Th-2 derived cytokines such as IL-10 (known to be implicated in CHS—see Kondo, McKenzie and Sauder, “The Journal of Investigative Dermatology,” Vol. 103, 1994, page 811-814) and / or down-regulates Th-1 inflammatory cytokines such as TNFα and IL-6. These inflammatory cytokines are implicated in inflammation-related disorders of the brain, namely the neuroinflammatory, neur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com