Implantable neuro-stimulation electrode with fluid reservoir
a neuro-stimulation electrode and fluid reservoir technology, applied in the field of drug eluting cochlear implant electrodes, can solve the problems of limiting the performance of the implant, difficult to predict the amount of trauma, and variable amount of connective tissue growth and trauma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
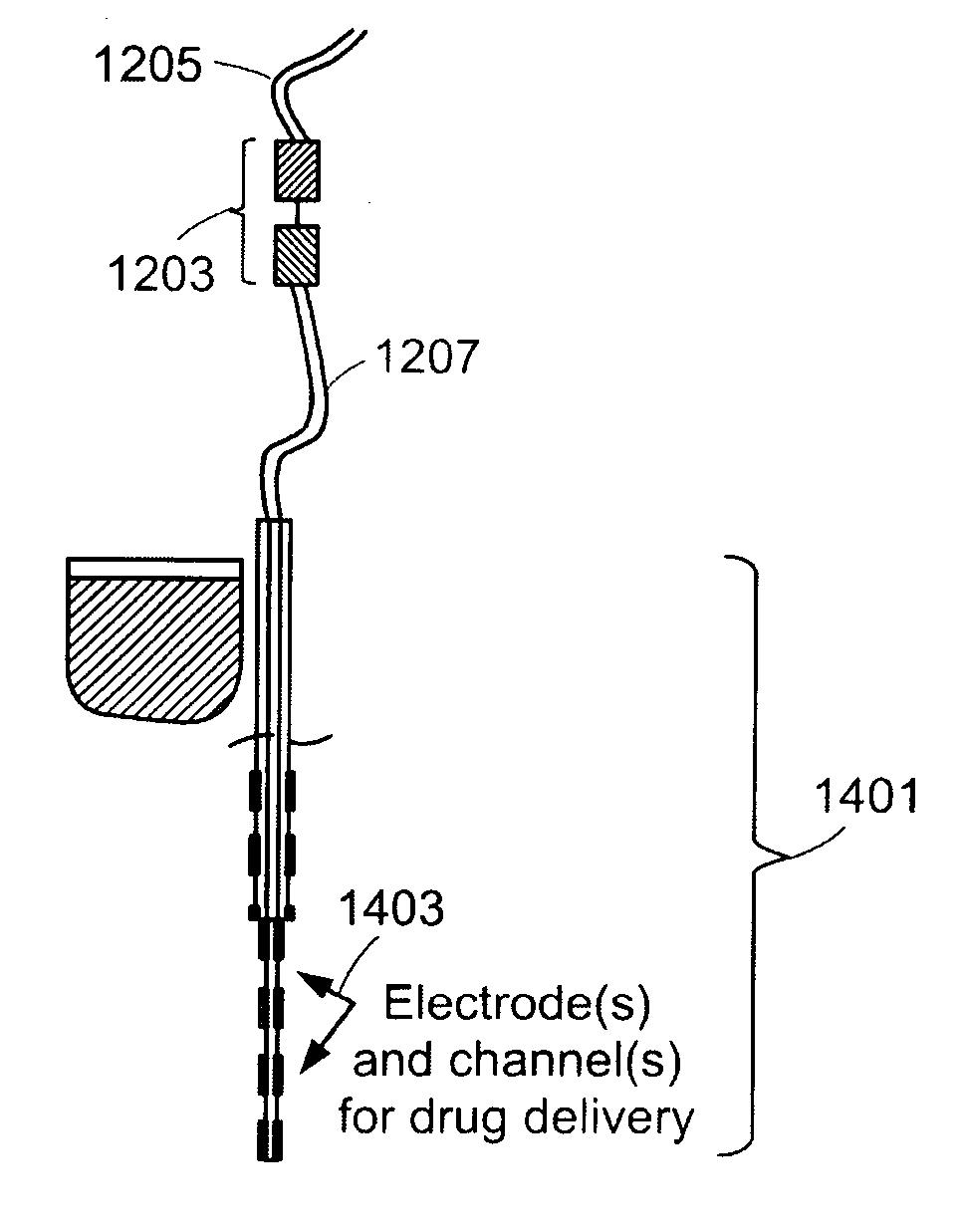
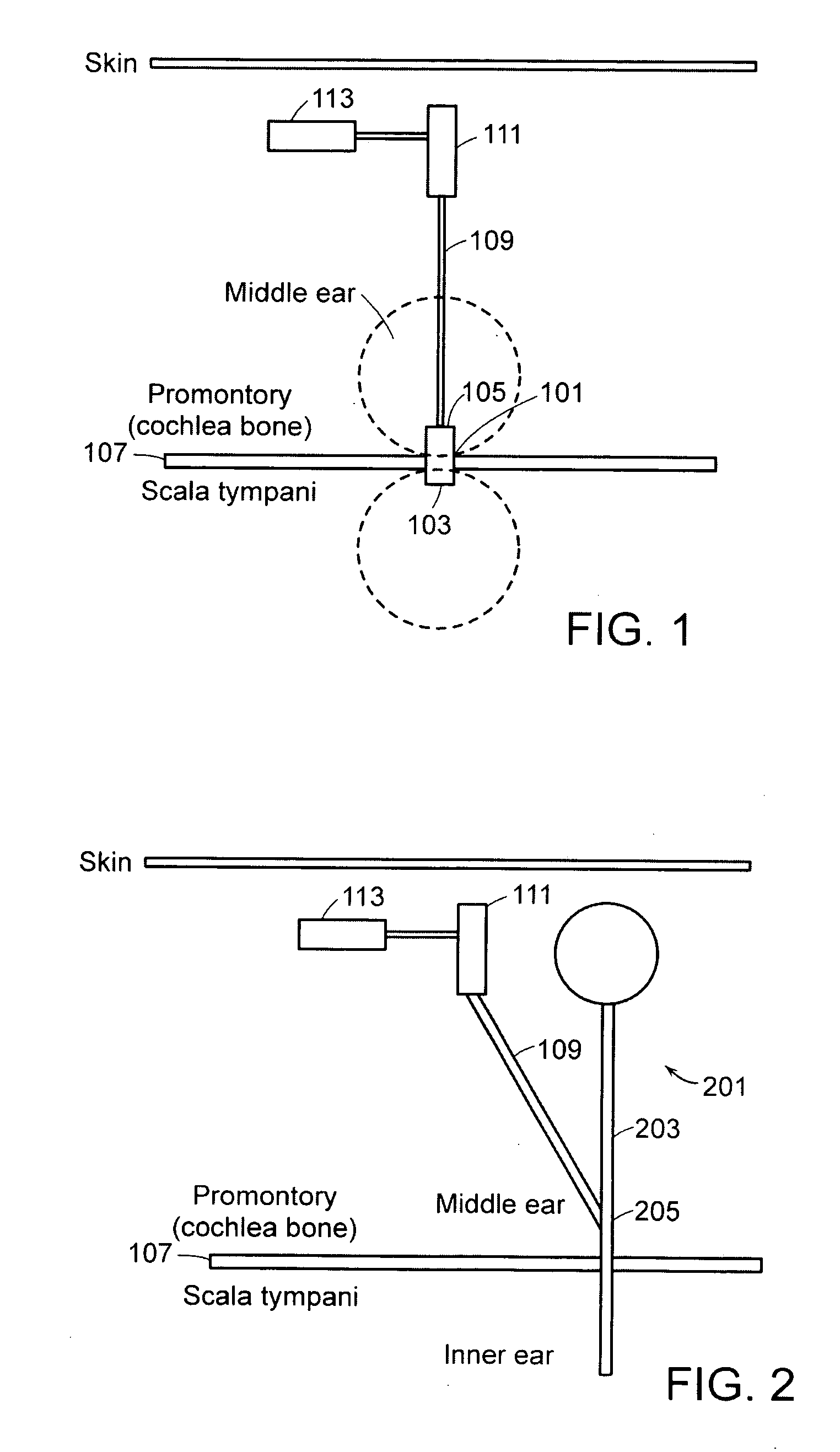
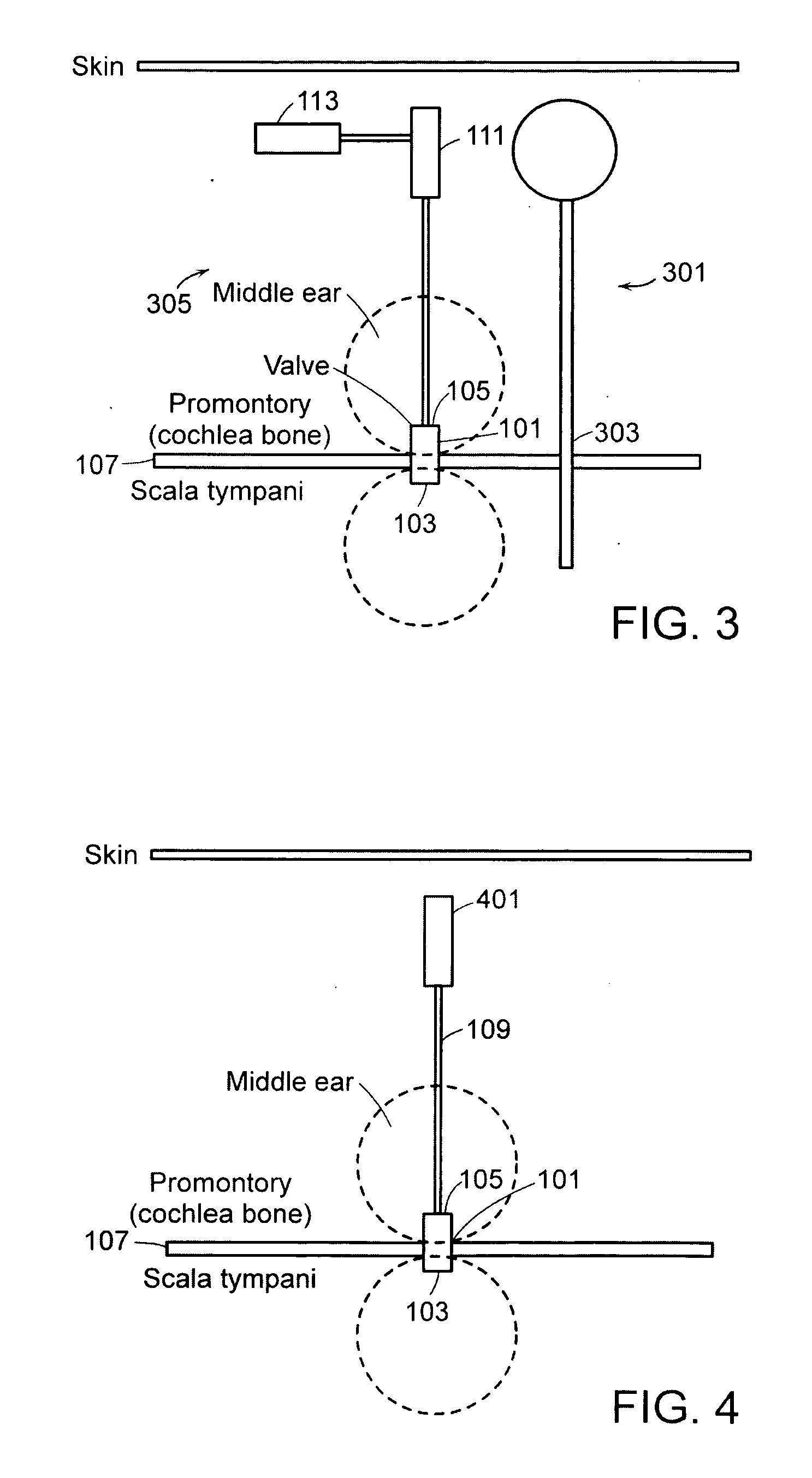
[0057]FIG. 1 is a graphical illustration of a fluid delivery system in accordance with an embodiment of the present invention. For purposes of this embodiment, the fluid delivery system is employed to deliver pharmaceuticals to, for example, the inner ear of a subject. However, the fluid delivery systems and apparatuses described herein may be used to deliver many different types of fluids to one or more internal areas of a subject's body. The system shown in FIG. 1 includes a biocompatible and sealed micro-valve 101 with an inner ear side 103 and a middle ear side 105. The micro-valve 101 provides a secure path between the middle ear and the inner ear through the promontory bone 107 of the cochlea or through the round window. The connection may, for example, be to the scala tympani, vestibuli or scala media. The micro-valve 101 provides permanent access to the inner ear for fluid delivery of various viscosity and healing functions. The micro-valve 101 may be made of, for example, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com