Medical Products and Parenteral Formulations
a technology of parenteral formulation and medical products, which is applied in the field of medical solutions and containers, can solve the problems of increasing the risk of contamination of the final mixture, easy to break, and difficult to handle, and heat sterilization such as autoclaving can affect certain plastic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
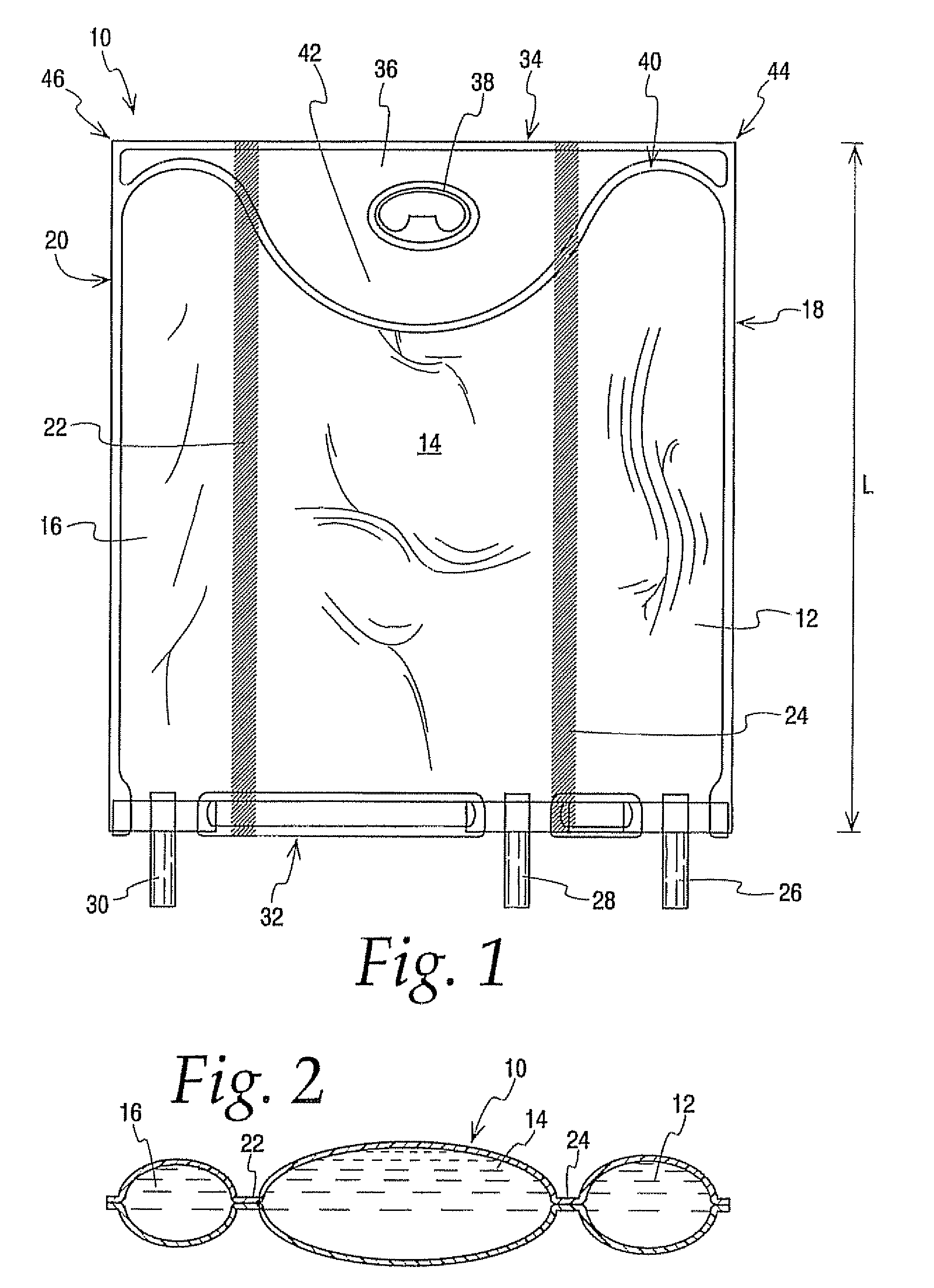
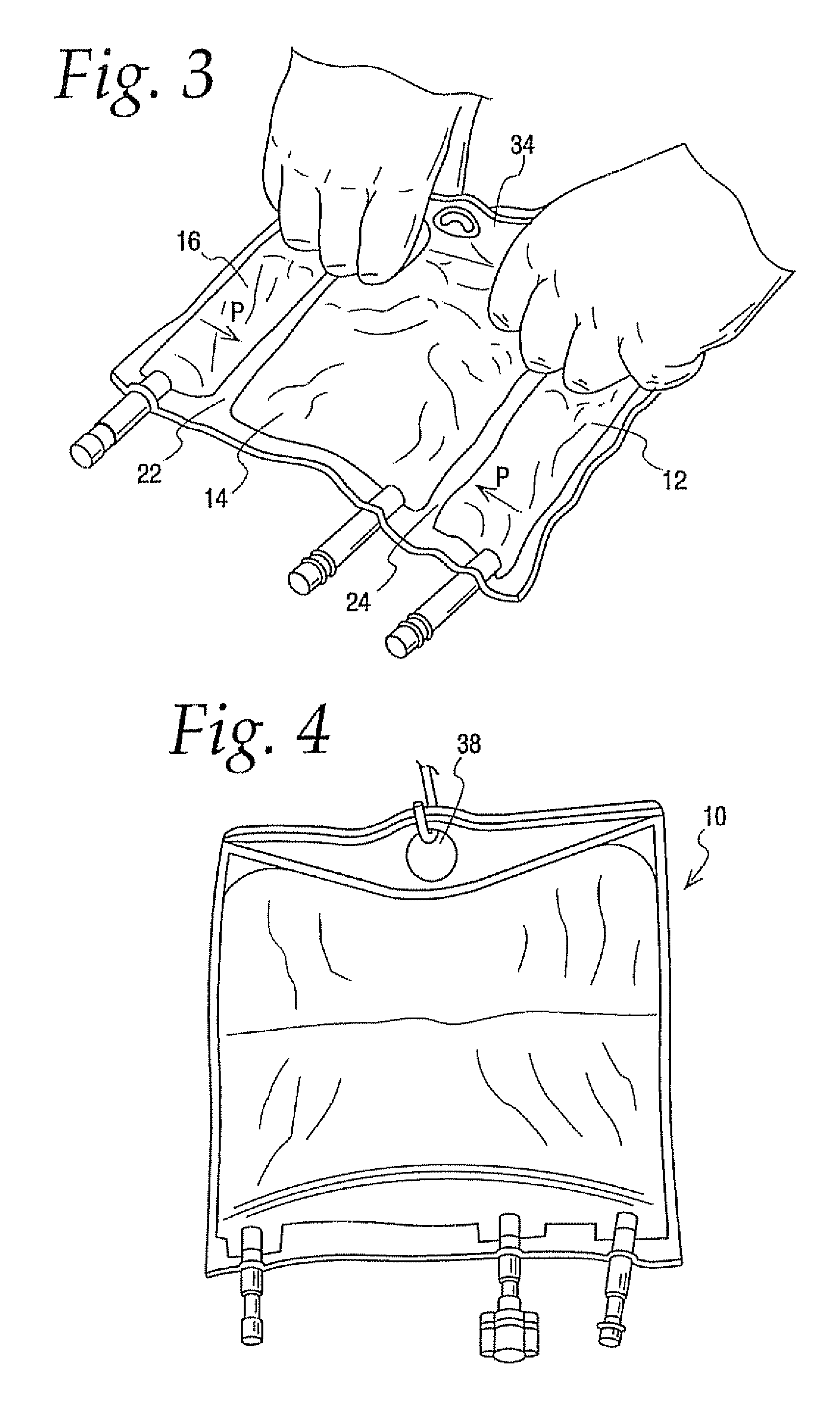
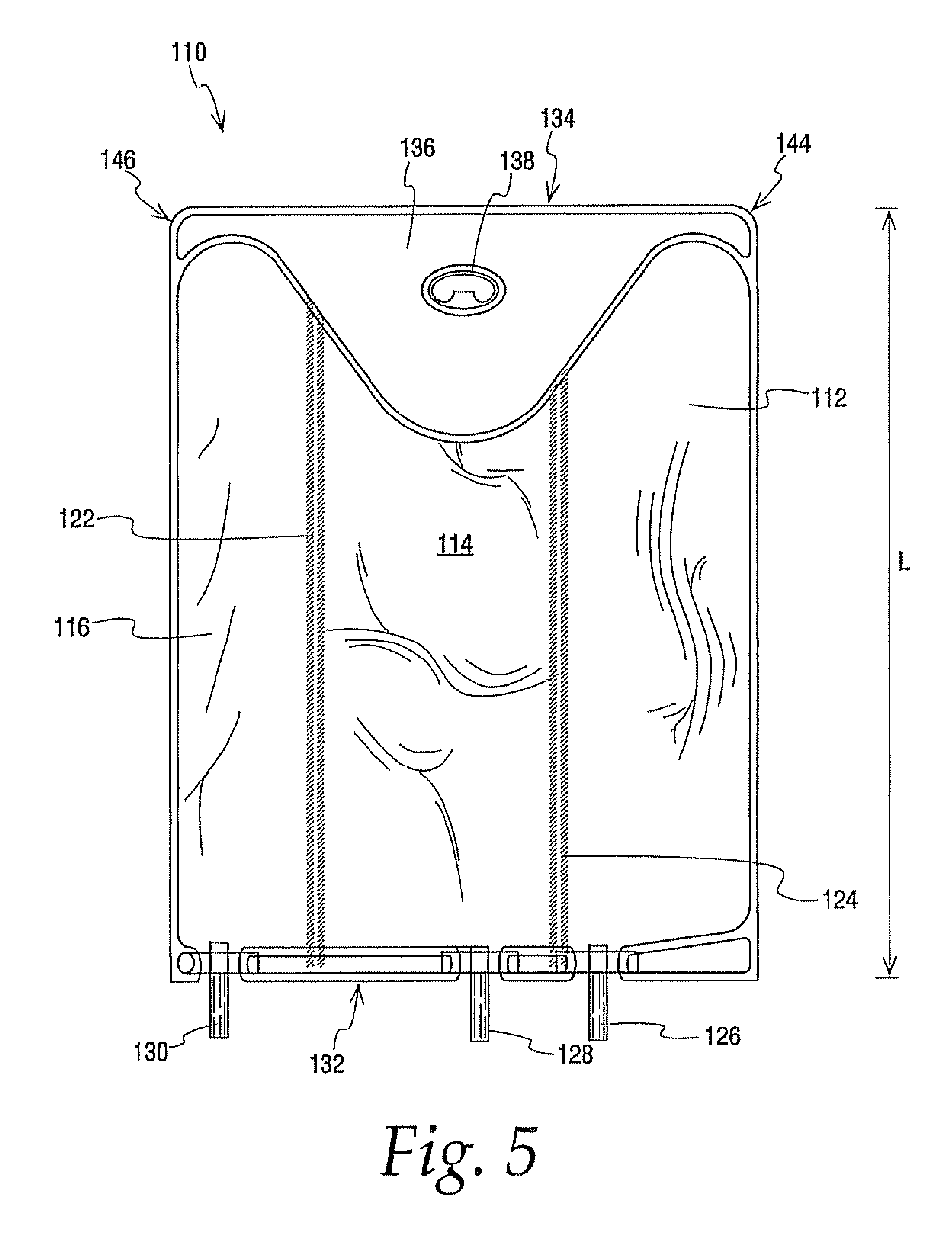
[0092] A comparison was of a 300 ml multi-chamber container of the present invention best exemplified by container 10 was compared to a currently available multi-chamber container which was the same in all respects to container 10 expect that the hanger flap extended only about half as far into the central chamber as hanger flap 36 extends into chamber 14 making the central chamber of this bag slightly larger in capacity. The same central and lateral end chambers were filled with water while the other lateral end chamber was filled with a colored solution. Additional water was added in the central chamber to compensate for the added volumetric capacity. In other words even though the central chamber of container 10 had a slightly smaller volume than the central chamber of other container they were similarly inflated with water.
[0093] Twenty operators were selected (10 male & 10 female). Each operator received 5 units of each design and the following instructions:
[0094] Instruction...
example 2
[0194] An indigo carmine indicator mixture was made as follows:
14 g indigo carmine, 60 g tetrasodium pyrophosphate, 2.75 g anhydrous dextrose, and 180 g microcrystalline cellulose were added to one liter of distilled water.
[0195] This mixture was placed in small pouches that were packed with oxygen absorber in an oxygen barrier overpouch and exposed to steam sterilization at 121° C. The samples were then stored in reduced form and the reduced form, i.e. yellow color of the indicator mixture, was still yellow after storage in a substantially oxygen free environment for 112 days at 50° C.
[0196] When similar packages were exposed to oxygen after being first placed in a reduced state as described above, the mixture changed to the oxidized form, i.e. dark blue color. The mixture remained dark blue after storage for 112 days at 50° C.
example 3
[0197] An indigo carmine indicator mixture was made as follows: 14 g indigo carmine, 60 g tetrasodium pyrophosphate, 2.00 g anhydrous dextrose and 180 g microcrystalline cellulose were added to one liter of distilled water. The results were similar to those found in Example 2 above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com