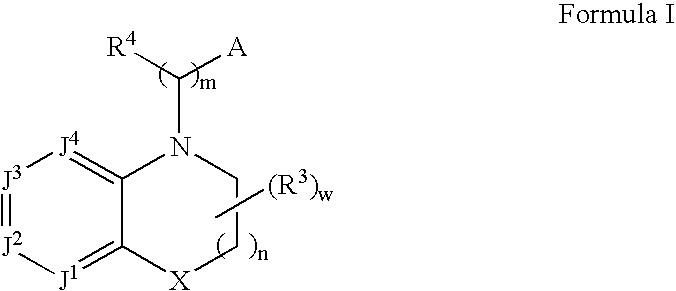
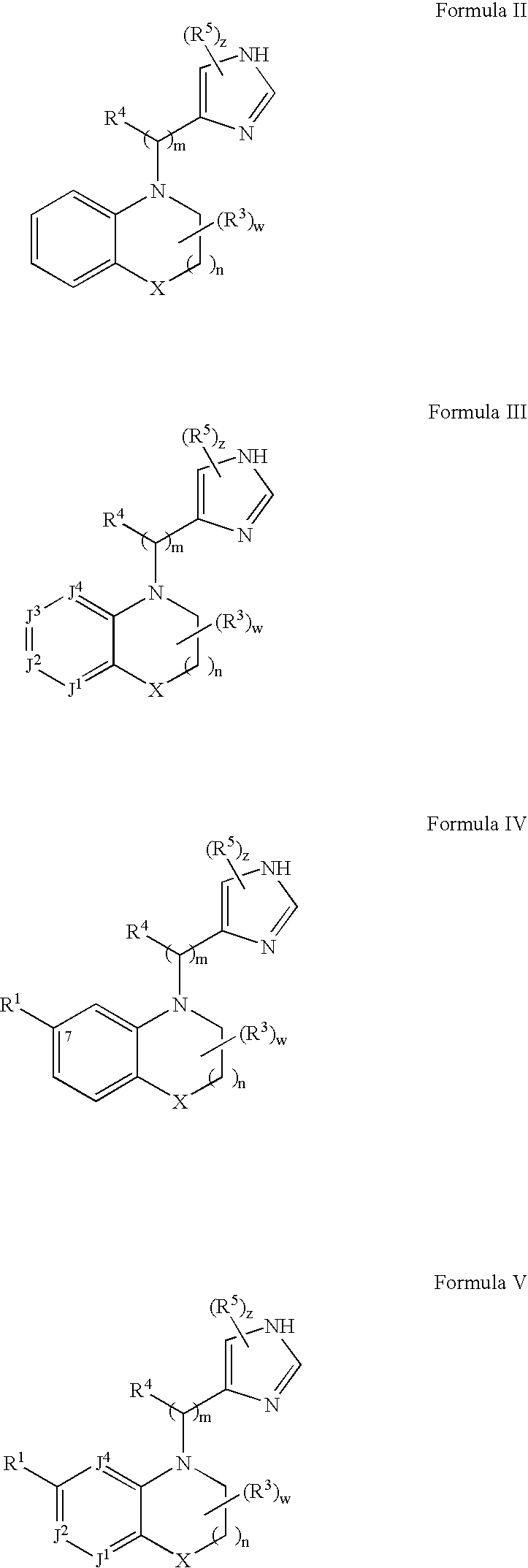
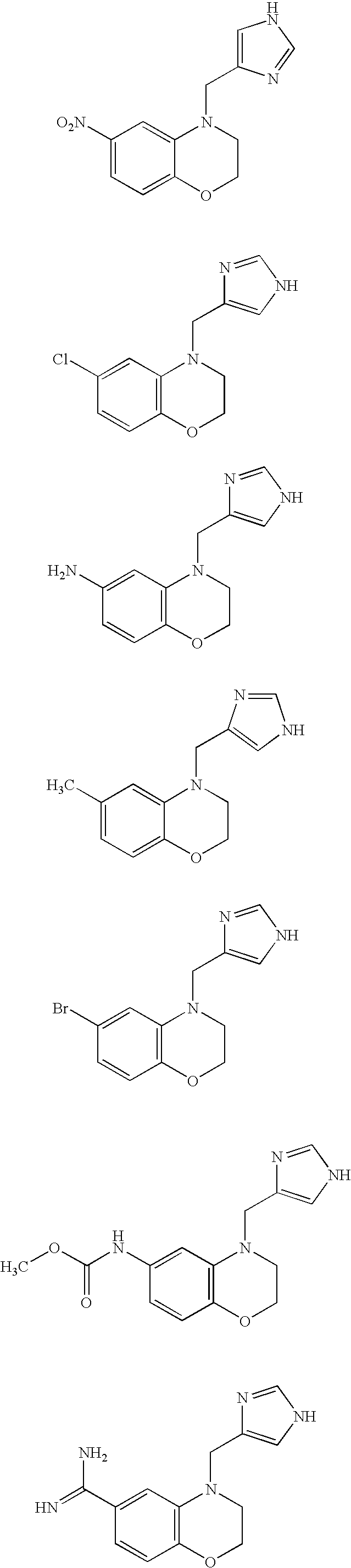
Alpha2C adrenoreceptor agonists
a technology of adrenergic agonists and alpha2c, which is applied in the field of alpha2c adrenergic agonists, can solve the problems that compounds having adrenergic activity, such as 2a agonists, may be associated with undesirable side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparative Example 1
[0205]
[0206] A solution of 3,4-dihydro-2H-1,4-benzoxazine 1A (0.1 g, 0.75 mmol) in DCE (10 mL) was treated with imidazole-4-carboxaldehye 1B (0.11 g, 1.1 mmol), NaBH(OAc)3 (0.47 g, 2.2 mmol), and ACOH (one drop) and stirred at 60° C. overnight. The reaction was then diluted with CH2Cl2, washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated. Chromatography (0-4% 7 N NH3-MeOH / CH2Cl2) provided 1 as a beige solid (0.08 g, 50%). LMCS m / z 216 (MH+).
[0207] Alternatively, the title compound 1 can be synthesized by the reaction of 1A and resin bound imidazole-4-carboxaldehye 1D as described below:
[0208] Novabiochem Resin 1C (100-200 mesh, 1% DVB, 1.4 mmol / g, 5 g) was suspended in anhydrous DMF (25 mL) and DCE (25 mL) and treated sequentially with 1B (2 g, 21 mmol) and TEA (2.96 mL, 21 mmol). The resin was shaken overnight and washed with DMF (3×), MeOH (3×), and DCM (4×) then dried in vacuo overnight. The resulting resin 1D (100 mg, 1.4 mmol / g, 0.1...
example 2
Preparative Example 2
[0209]
[0210] A solution of 3,4-dihydro-2H-1,4-benzoxazine (0.52 g, 3.8 mmol) and thiophene-3-acetic acid (0.82 g, 5.7 mmol) in 1:1 CH2Cl2:DMF (20 mL) was treated with DIPEA (2.6 ml, 15 mmol), HOBt (1.29 g, 9.5 mmol), and EDCI (1.83 g, 9.5 mmol) and stirred at 70° C. overnight. The reaction was then diluted with CH2Cl2, washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated. Chromatography (0-10% 1 N NH3-MeOH / EtOAc) provided 2A as a red solid (0.54 g, 55%)
Step 2
[0211] A solution of 2A (0.094 g, 0.36 mmol) in THF (10 mL) was treated with BH3—SMe2 (2M / THF, 0.27 mL, 0.54 mmol) and stirred at reflux for 2 h. The reaction was concentrated and subjected to chromatography (EtOAc) to provide 2 as a white solid (0.040 g, 45%). LMCS m / z 246 (MH+).
example 3
Preparative Example 3
[0212]
[0213] A mixture of 2-amino-4-nitrophenol (3A, 25.03 g, 0.16 mol) in 4-methyl-2-pentanone and water (420 mL, 1:1) was treated with sodium bicarbonate (32.74 g, 0.39 mol), cooled to 0° C., and treated then with chloroacetyl chloride (15.52 mL, 0.19 mol). The reaction mixture was heated to reflux overnight. After cooling to RT, the mixture was concentrated under vacuum. The residue was diluted with water (200 mL) and EtOAc (100 mL), and filtered to give the pale gray solid 3B (26.05 g). The filtrate was separated and the aqueous was extracted with EtOAc (3×100 mL). The combined organic layers were washed with water, and dried (MgSO4), filtered, and concentrated under vacuum to give additional light gray solid 3B (7.7 g). The resulting solid (quantitative yield) was used for next reaction without further purification.
[0214] To compound 3B (6.76 g, 34.84 mmol) in anhydrous THF (200 mL) was added BH3—SMe2 (2.0M / THF, 35 mL, 69.68 mmol). The mixture was heated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com