Use of modified extracellular matrix proteins in diagnosis and treatment of atherosclerosis
a technology of extracellular matrix protein and diagnosis, applied in the direction of antibody medical ingredients, peptide/protein ingredients, instruments, etc., can solve the problems of fragmentation and aldehyde-modification of respective matrix proteins, and remain largely unexplored
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
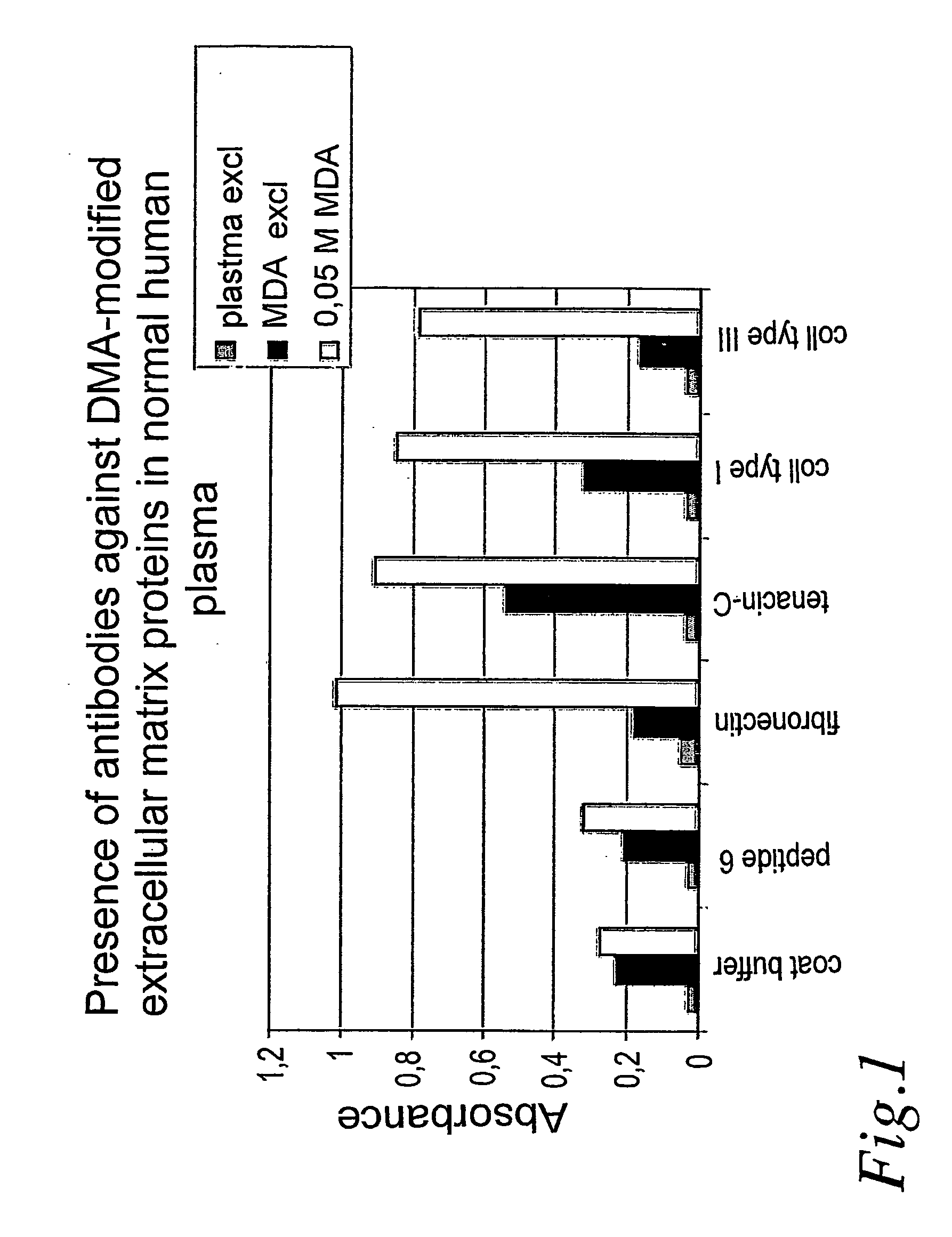
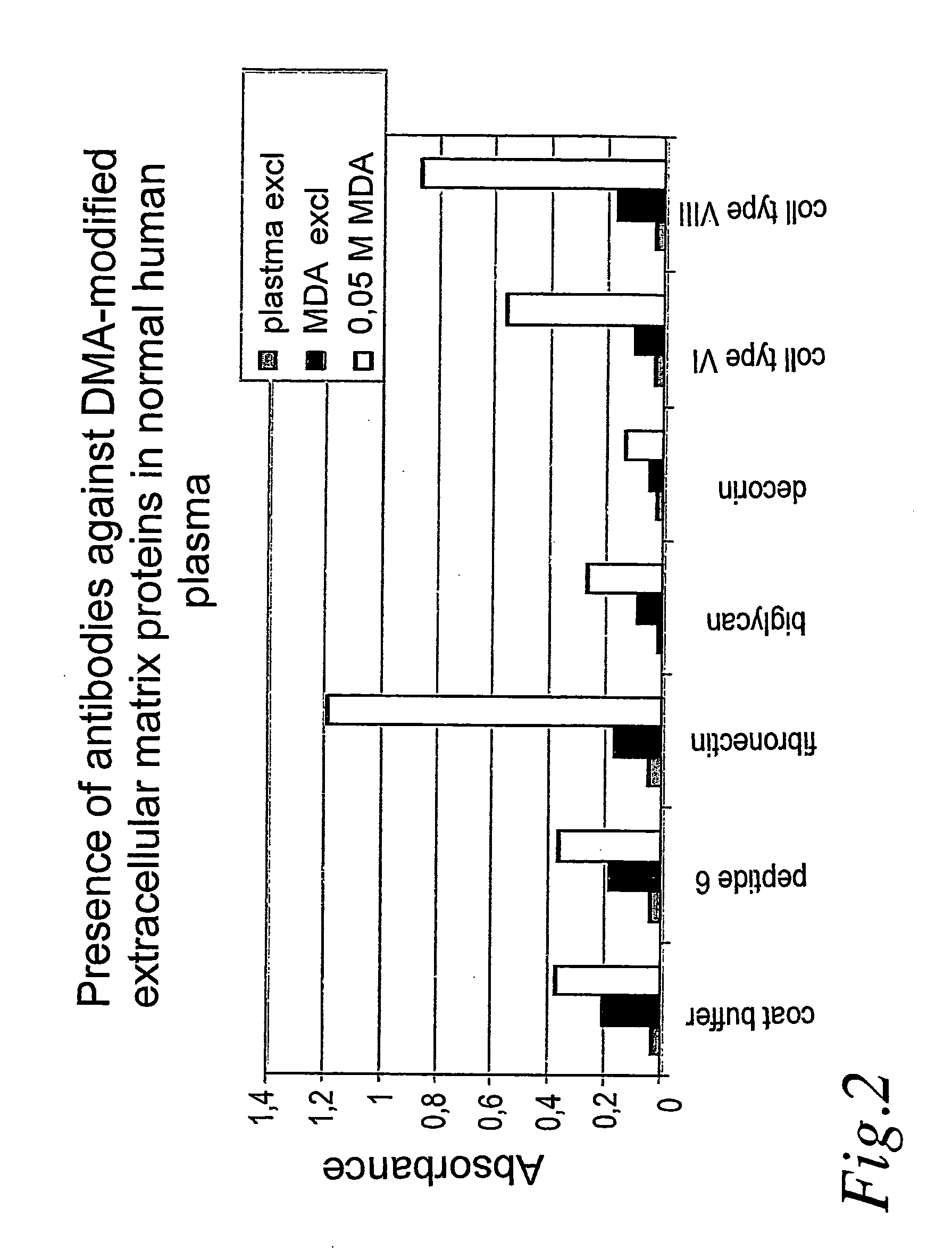
[0009] The present invention in particular relates to the use of fibronectin, tenascin, collagens type I, III, VI and / or VIII modified by aldehyde or by glycosylation in ELISA for detection of antibodies in plasma and serum to diagnose atherosclerosis.
[0010] In a preferred embodiment the fibronectin, tenascin, collagens type I, III, VI and VIII have been modified by malondialdehyde.
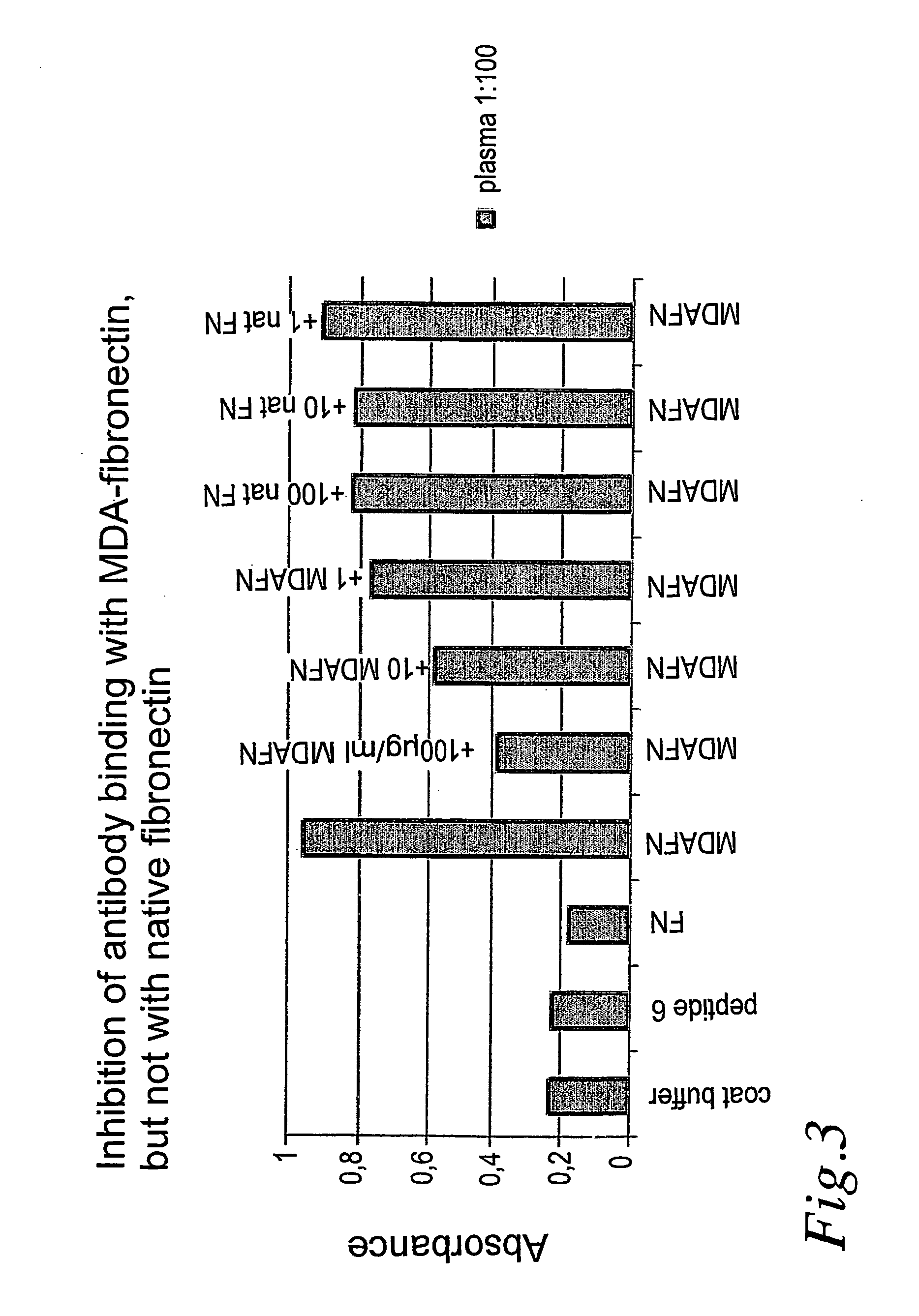
[0011] A further preferred embodiment relates to the use of antibodies against glycosylated or aldehyde-modified fibronectin, tenascin, collagens type I, III, VI and VIII produced by immunization or recombinant technique for detection of aldehyde-modified or glycosylated fibronectin, tenascin, collagen type I, III, VI and / or VIII in plasma and serum to diagnose presence of unstable and rupture-prone atherosclerotic plaques.
[0012] A still further preferred embodiment relates to the use of labelled antibodies against glycosylated or aldehyde-modified fibronectin, tenascin, collagens type I, III, VI and / o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com