TRANSGENIC NON-HUMAN MAMMAL WITH AN ONCOGENIC MUTANT OF THE c-Raf-1 GENE
a technology of c-raf-1 and non-human mammal, which is applied in the field of transgenic non-human mammals, can solve the problems of little success of therapy predictions, and achieve the effects of reducing alveolar surface tension, high selectivity and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
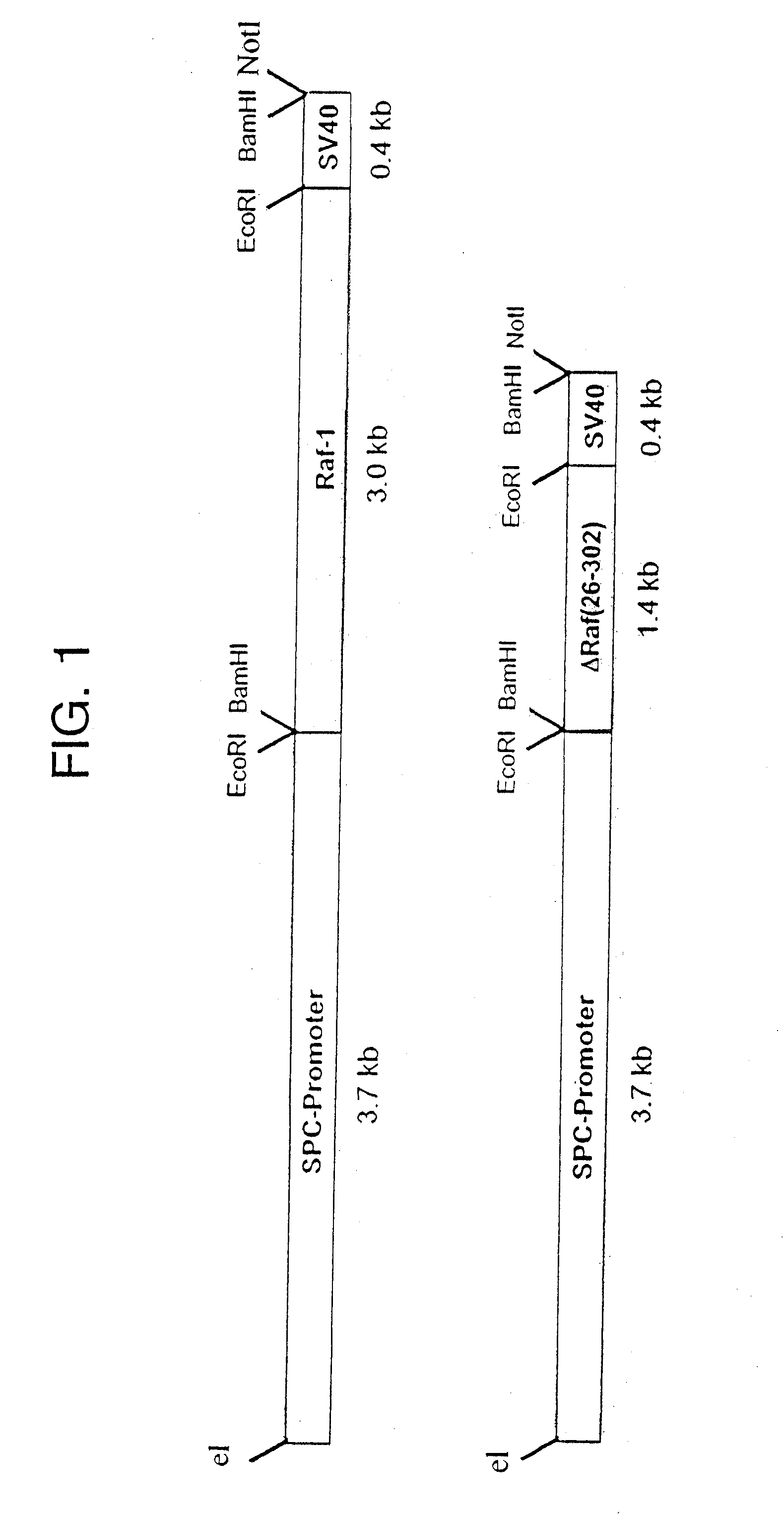
[0029] Cloning of the cDNA sequences of the transforming c-Raf subdomain into a lung-specific expression vector was performed as follows. The vector SPC-Raf-1 for generating the wild type-Raf-1 transgenic mice was produced by that a 3.0 kb fragment of the human Raf-1 cDNA (Bonner, T. I.; Oppermann, R.; Seebrug, P.; Kerby, S. B.; Gunnell, M. A.; Young, A. C.; and Rapp, U. R.; 1986; “The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gen.”; Nucleic Acids Res.; 14, 109) was cloned in the EcoRI interface of the plasrid SPC3.7 / 5V40 including the 3.7 kb promoter region of the human surfactant-associated protein C(SPC) (Korfhagen, T. R.; Glasser, S. W., Wert, S. E. Bruno, M. D.; Daugherty, C. C.; McNeish, J. D.; Stock, J. L.; Potter, S. S.; Whitsett, J. A.; 1990; “Cis-acting sequences from a human surfactant protein gene confer pulmonary-specific gene expression in transgenic mice.”; Proc. Natl. Acad. Sci.; 87, 6122). In analogous manner, ...
example 2
[0030] The linearization and the pronucleus injection were performed as follows. The transgenic vectors were cut with the restriction endonucleases NotI and NdeI, cleaned with a preparative agarose gel (Sambrooks et al., 1989, see below), and diluted to a concentration of 1 ng / ml. 200 ng of the linearized DNA fragments were injected into the male pronuclei of fertilized oocytes. Transgenic founder mice were identified by analysis of the genomic DNA isolated from tail ends by Southern Blot (see also example 3). The founder mice were crossed with non-transgenic B6D2 mice, in order to establish stable lines.
[0031] The used mice were C57BL / 6DBA F2 mice (B6D2 mice), and were obtained from Harlan Winkelmann (Borchen) and from Charles River (Sulzfeld), and were held and bred on in the stable of the MSZ (Institut für Medizinische Strahlenkunde and Zellforschung, Würzburg University, 097078 würzburg) under pathogen-free conditions.
example 3
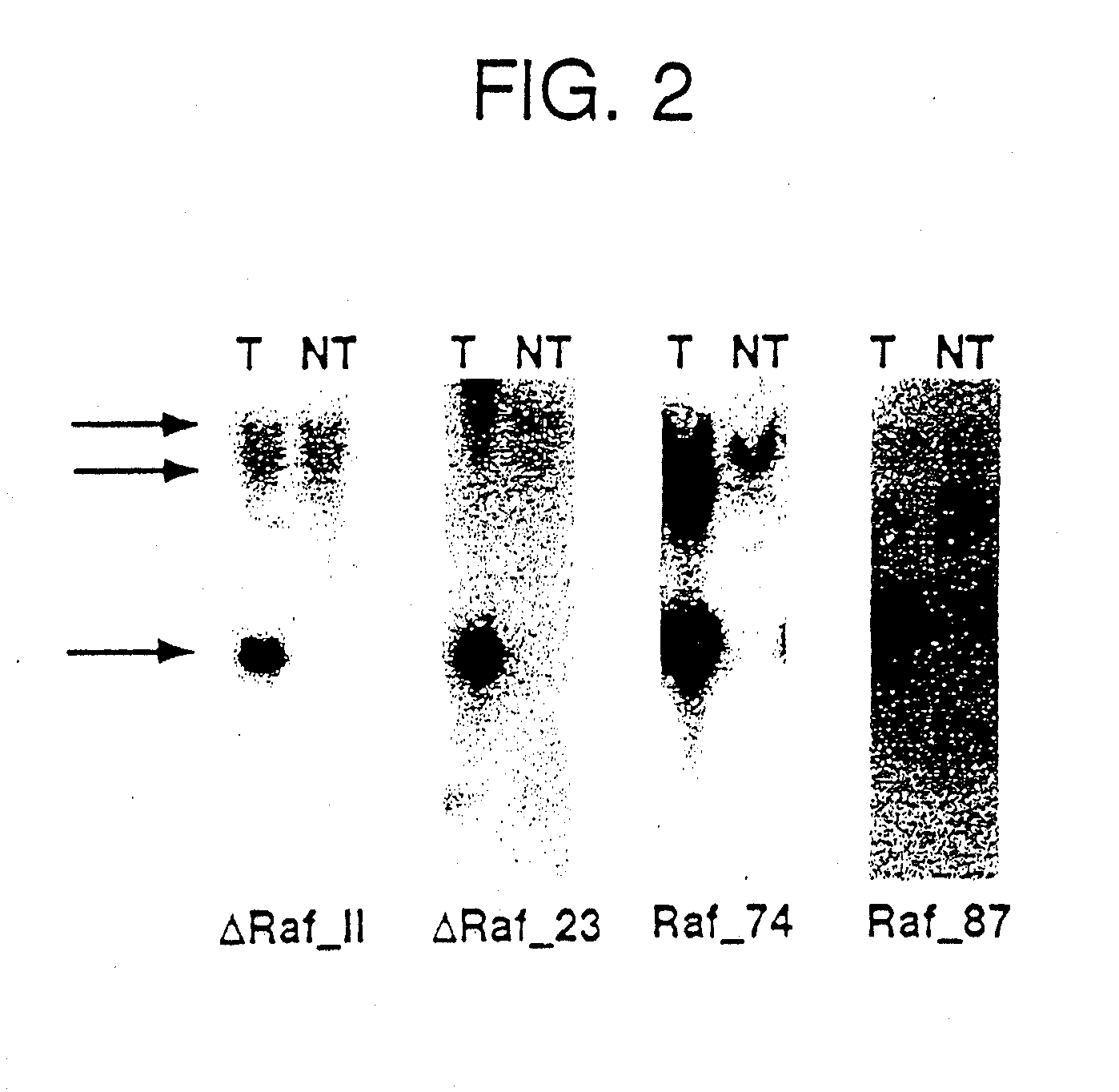
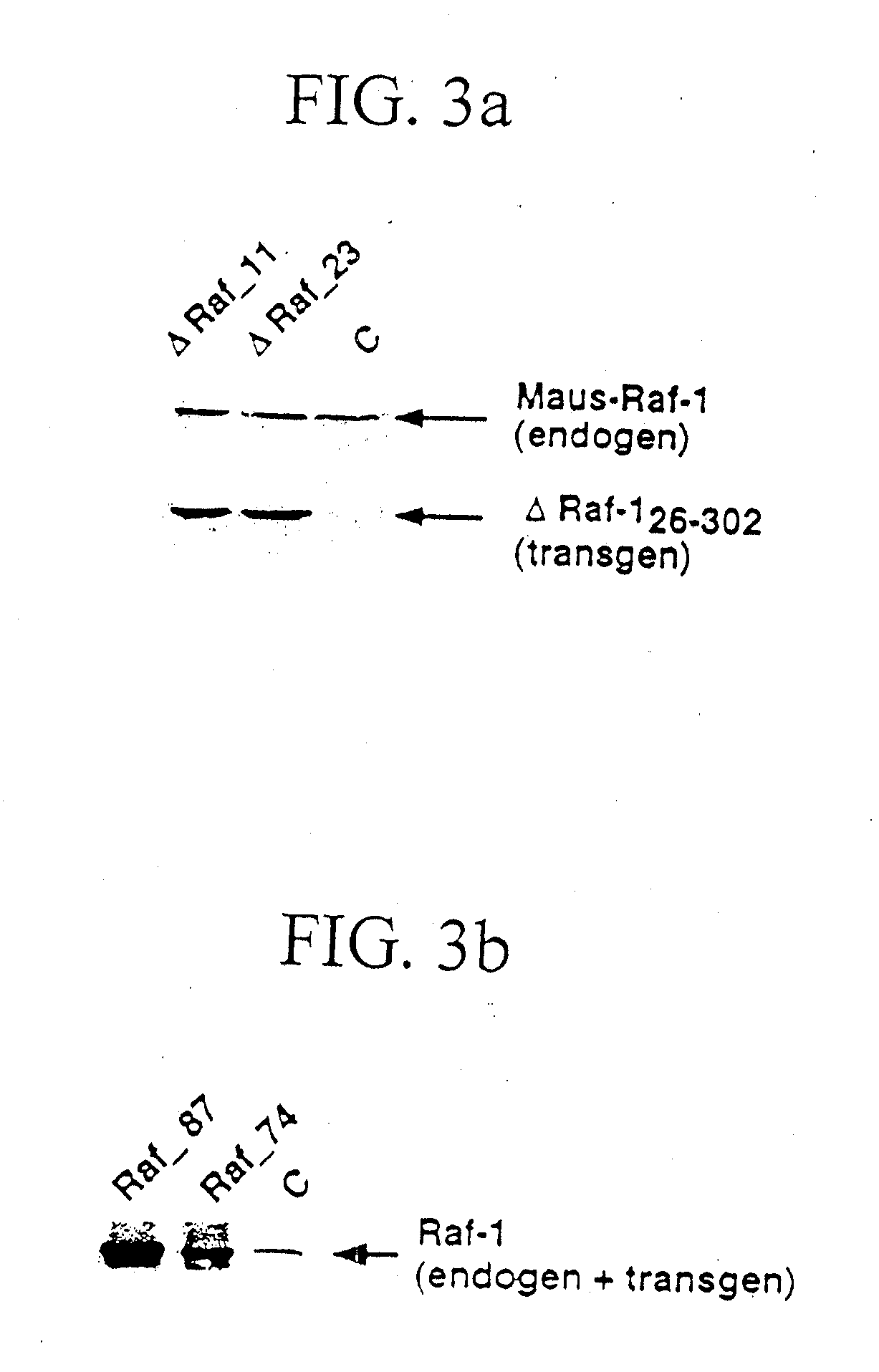
[0032] PCR and Southern Blot for genotypization were performed by the following operational instructions. For the detection of the transgenic integration, 10 μg genomic DNA from tail ends were cut over night with 40 units BamHI, separated on a 0.7% agarose gel by means of electrophoresis, and transferred by means of a capillary blot to nitrocellulose (Sambrook, J.; Fritsch, E. F.; Maniatis, T.; “Molecular Cloning: a laboratory manual)”; Cold Spring Harbor Laboratory Press). After fixation of the DNA by u.v. light followed the detection of the transgene by hybridization of the membrane (Church, G. M. and Gilbert, W.; “Genomic sequencing”; Proc. Natl. Acad. Sci.; 81, 1991) with a Raf-1 probe (containing the Raf-1 / SV40 sequence) that was obtained by digestion of the transgenic vector SPC-Raf-1 with BamHI. The bound probe cross-hybridizing also with the mouse-Raf locus, was detected by exposure of the membrane on film material, and marked a 3.4 kb Raf 1 / SV40 fragment or a 1.8 kb ΔRaf (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| structures | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com