Methods for determining the prognosis for cancer patients using TUCAN
a technology of prognosis and cancer, applied in the field of cancer, can solve the problems of less reliable diagnosis of certain markers, profoundly affecting the more than 1 million people, and their families and friends
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Generation of Antibodies for Immunodetection of IAPs and Apaf1
[0141] This example shows preparation and characterization of antibodies useful for detecting IAPs and Apaf1.
[0142] Antisera were raised against recombinant proteins and synthetic peptides for immunodetection of Survivin, XIAP, Apaf1, AIF and Smac. Prior to employing these antibodies for analysis of cancers, the specificity of these antibodies for their intended protein targets was confirmed by SDS-PAGE / immunoblot analysis. Examples of data are provided in FIG. 3. FIG. 3A shows in vitro translated Survivin, XIAP, cIAP1, cIAP2, NAIP, BRUCE, and baculovirus Cp-IAP proteins were subjected to SDS-PAGE / immunoblot analysis, using polyclonal XIAP antiserum (AR-27A). Incubation with XIAP antiserum detected only XIAP in vitro translated protein. Detergent lysates were prepared from various normal human tissues, normalized for total protein content (50 ug), and subjected to SDS-PAGE / immunoblot assay using antisera specific for Su...
example ii
Immunohistochemical Analysis of IAPs and Other Biomarkers in Normal Colonic Mucosa and Colon Cancer
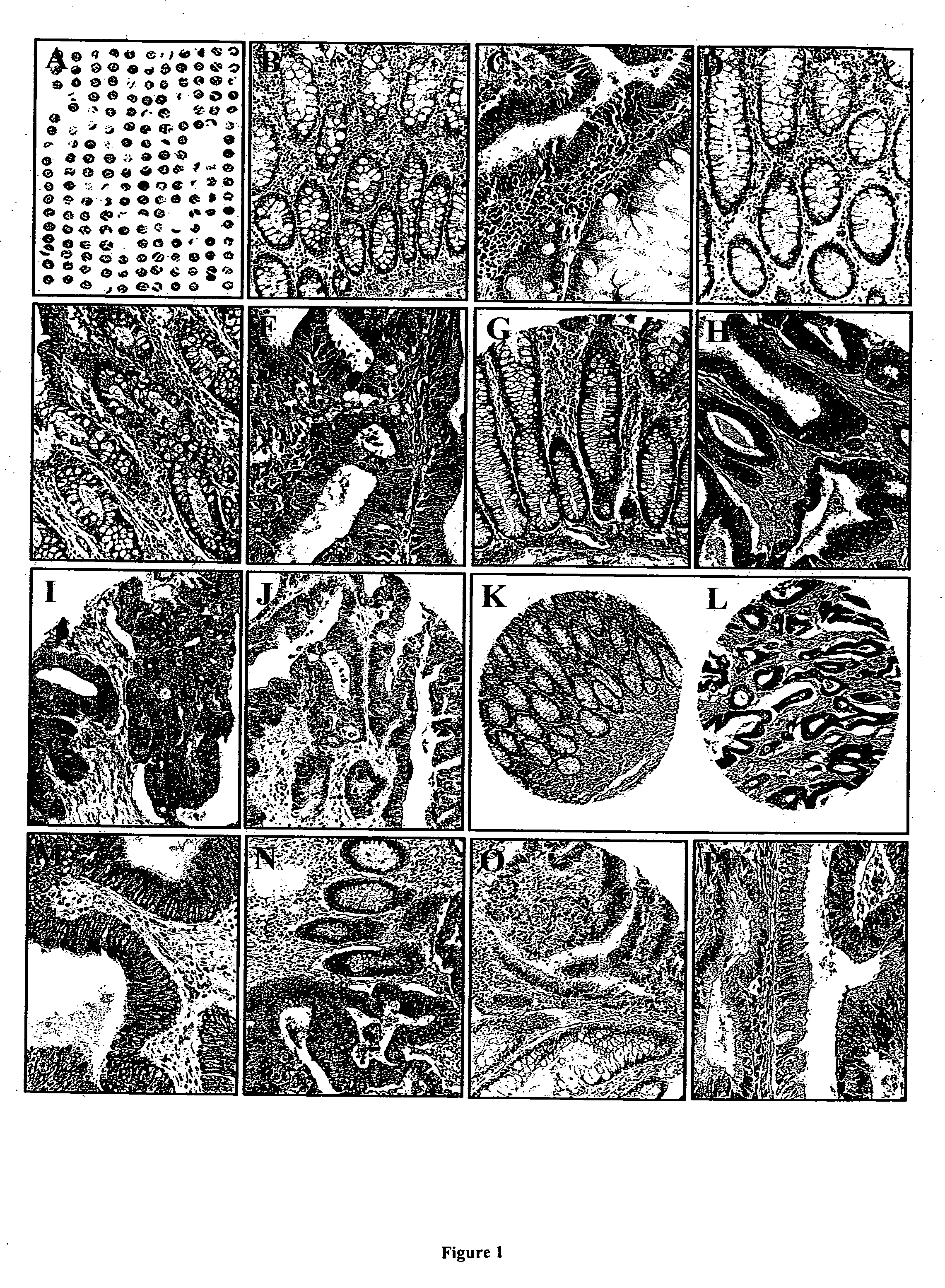
[0145] This example shows immunohistochemical analysis of IAPs and other biomarkers in a tissue microarray representing tissue samples obtained from 102 individuals.
[0146] A tissue microarray was constructed using primary tumor specimens derived from a relatively homogenous cohort of 102 patients presenting with stage II disease (Dukes' B stage) to a single institution, and who were treated by surgical resection with curative intent. Colon carcinoma specimens were obtained from Department of Pathology, Yonsei University, College of Medicine, Seoul, Korea. Samples were taken from primary tumors derived from patients who presented between 1986 and 1996 with Dukes' B stage [stage II disease, as defined by American Joint Committee on Cancer and Union Internationale Contre le Cancer (AJCC / UICC) criteria]. Patients with Dukes' stage B2 (T3N0M0) constituted 91% of the cohort, whereas 9% suf...
example iii
Immunoblot Analysis of IAPs and Apaf1 in Colon Carcinoma
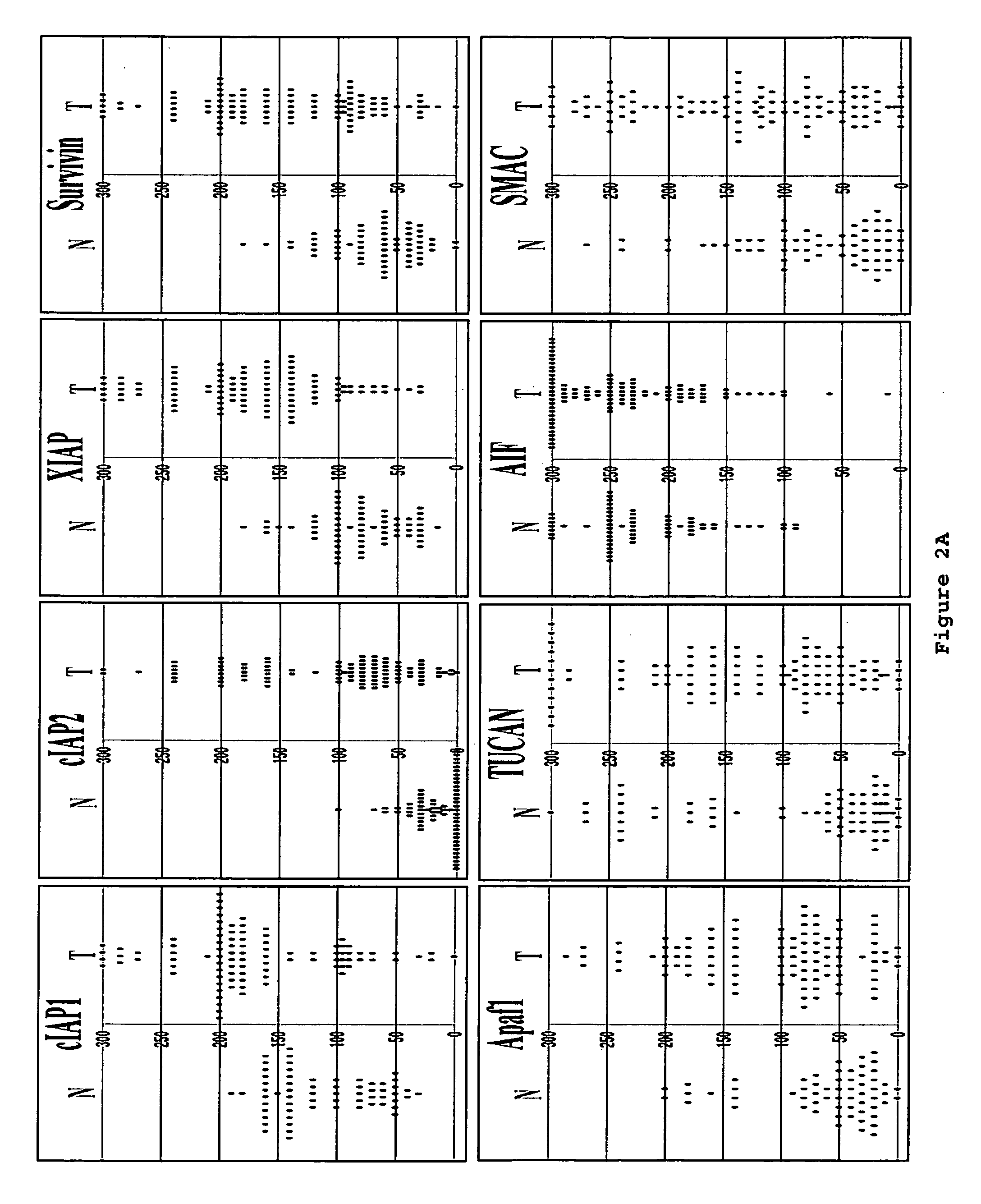
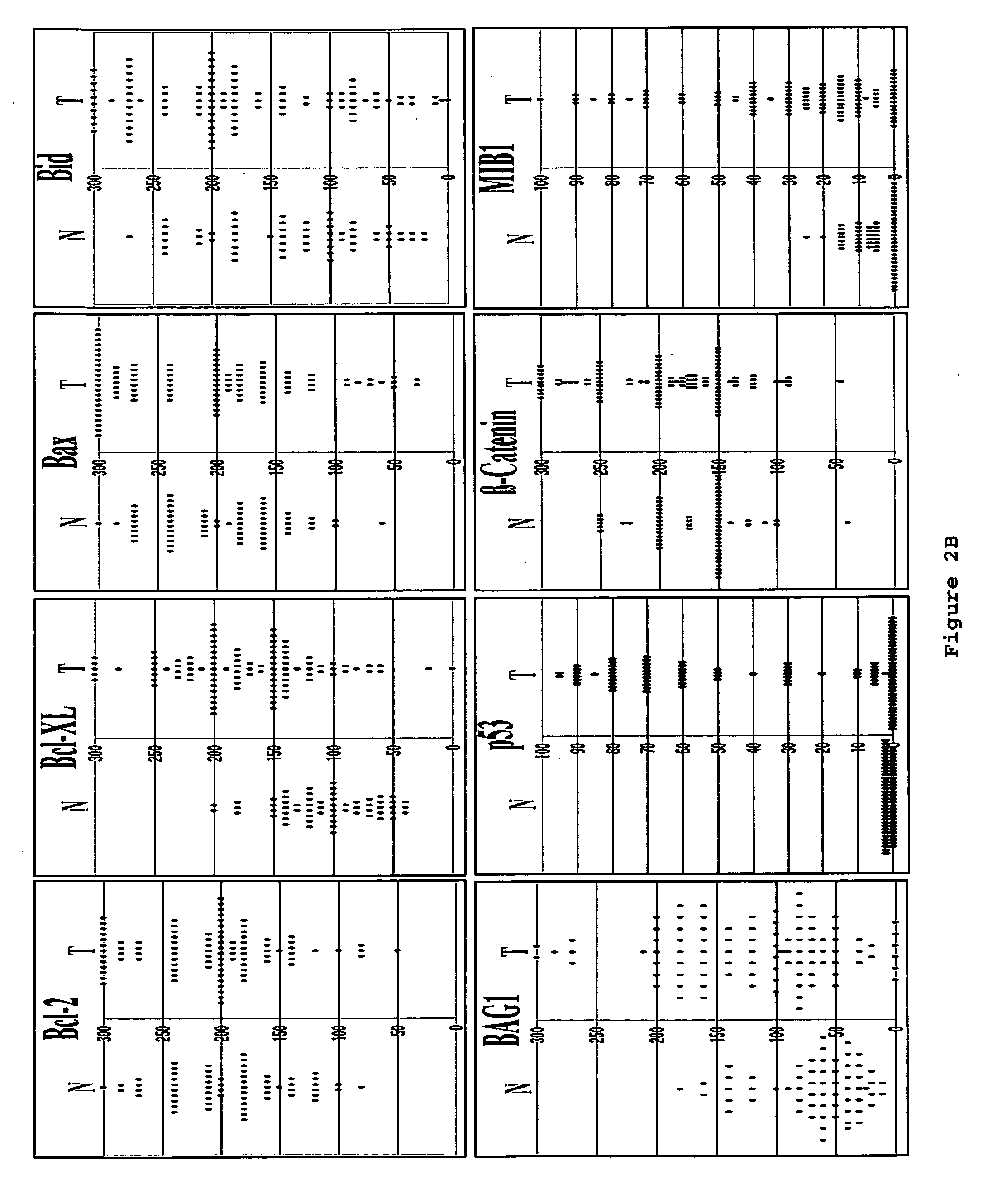
[0153] This example shows immunoblot analysis of IAPs, Apaf1 and other apoptosis-regulators in five frozen colon cancer specimens.
[0154] To corroborate the immunohistochemistry data, five frozen colon cancer specimens were identified that had sufficient amounts of both adjacent normal (N) and tumor (T) tissue for immunoblot analysis using antibodies specific for IAPs, Apaf1, and other proteins. Detergent-lysates of these tissues specimens were prepared and normalized for total protein content prior SDS-PAGE / immunoblot analysis (FIG. 3E). Densitometry analysis was also performed to quantify band intensities, and the results from the loading control blot were used to normalize all data (FIG. 3F).
[0155] Colon cancer specimens (n=10) with high ratios of cancer cells relative to stroma (>70%) were selected for immunoblotting analysis. The protein lysates were prepared without additional microdissection or fractionation. The tumor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| computed tomography | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com