Modulation of Cartilage Homeostasis By Active Domains of Cell Binding Extracellular Matrix Molecules
a cell binding extracellular matrix and active domain technology, applied in the direction of animals/human proteins, cyclic peptide ingredients, animals/human peptides, etc., can solve the problem of unfavorable side effects and the lik
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
[0130] The following examples are intended to illustrate the invention without limiting it thereto.
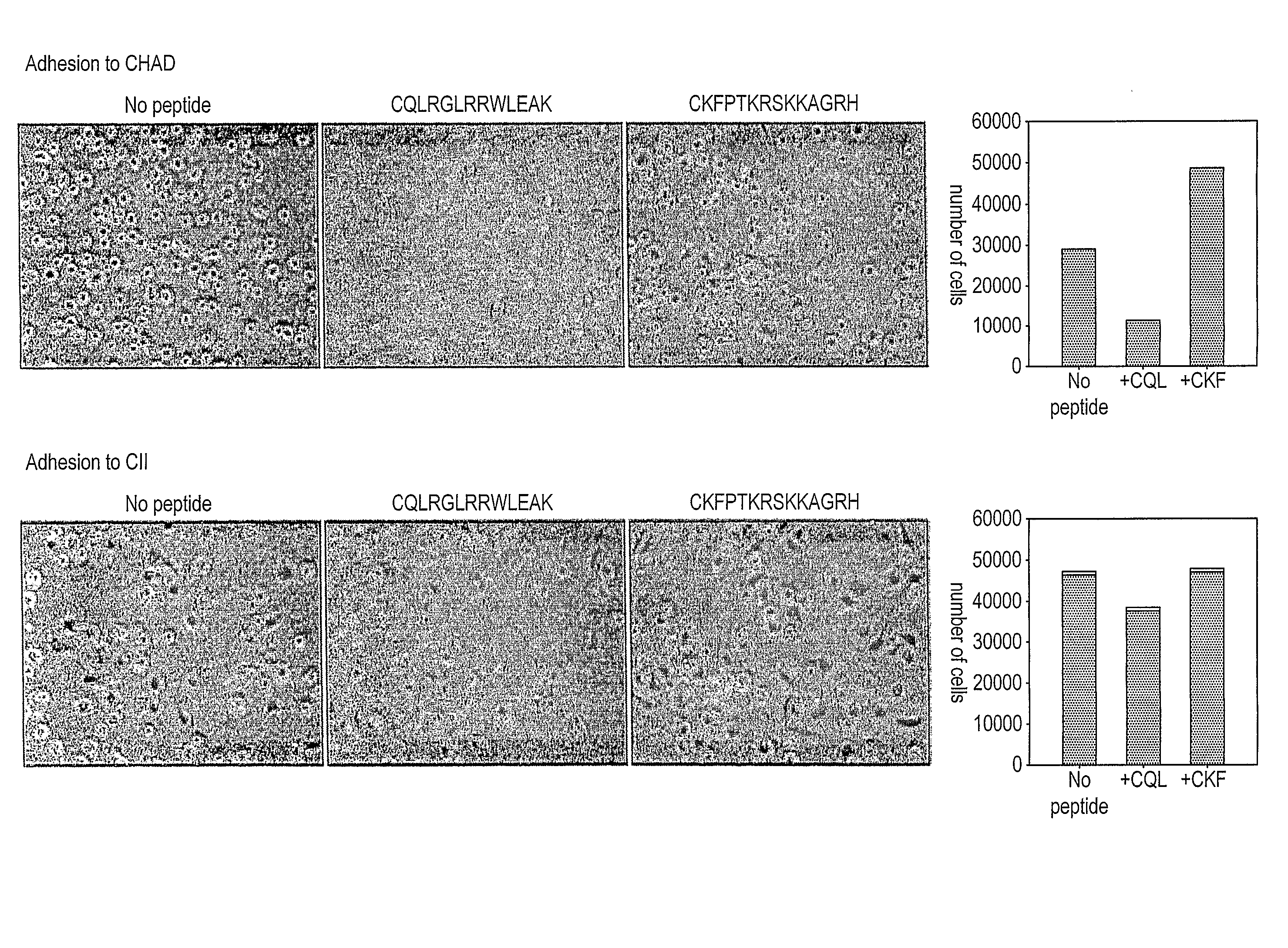
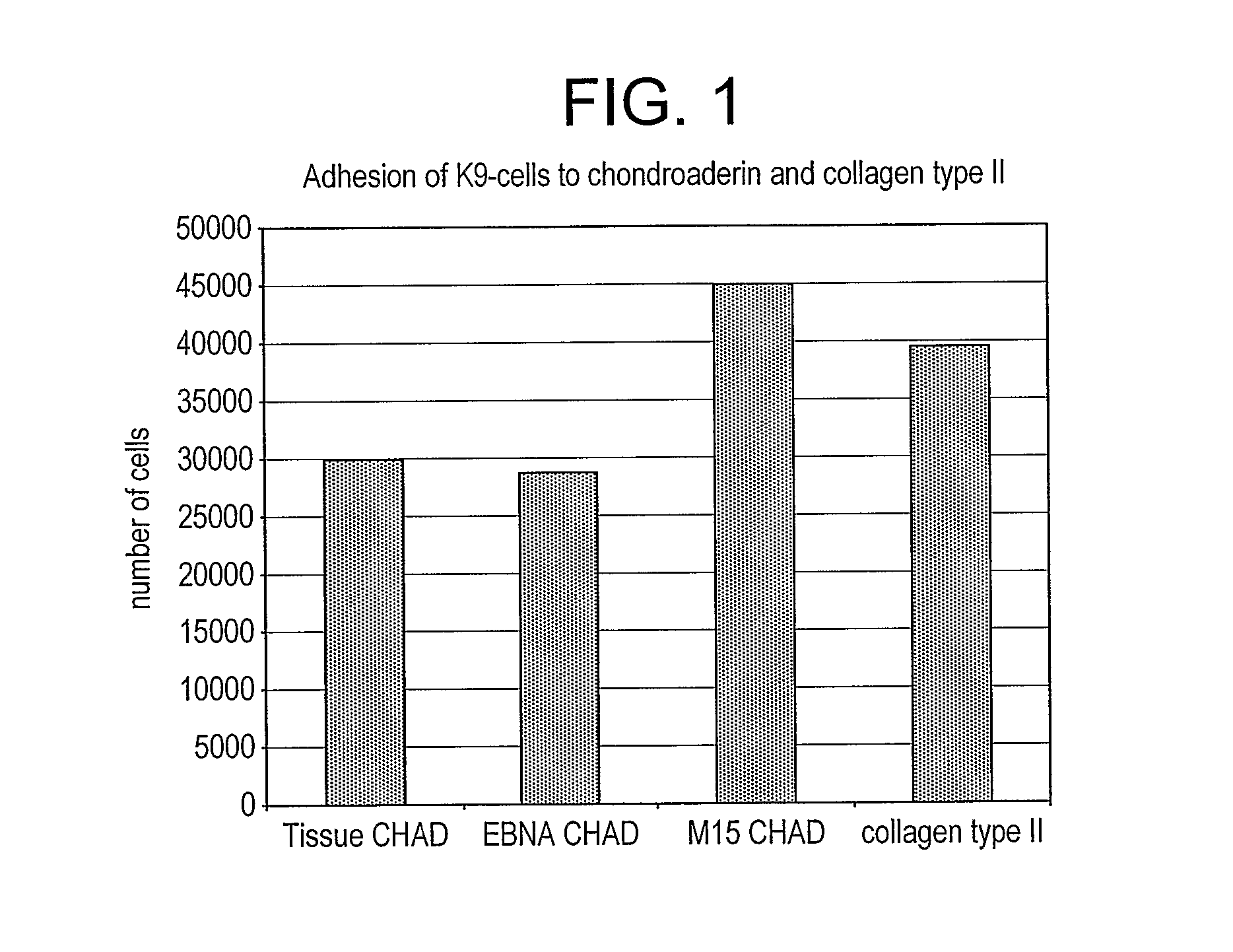
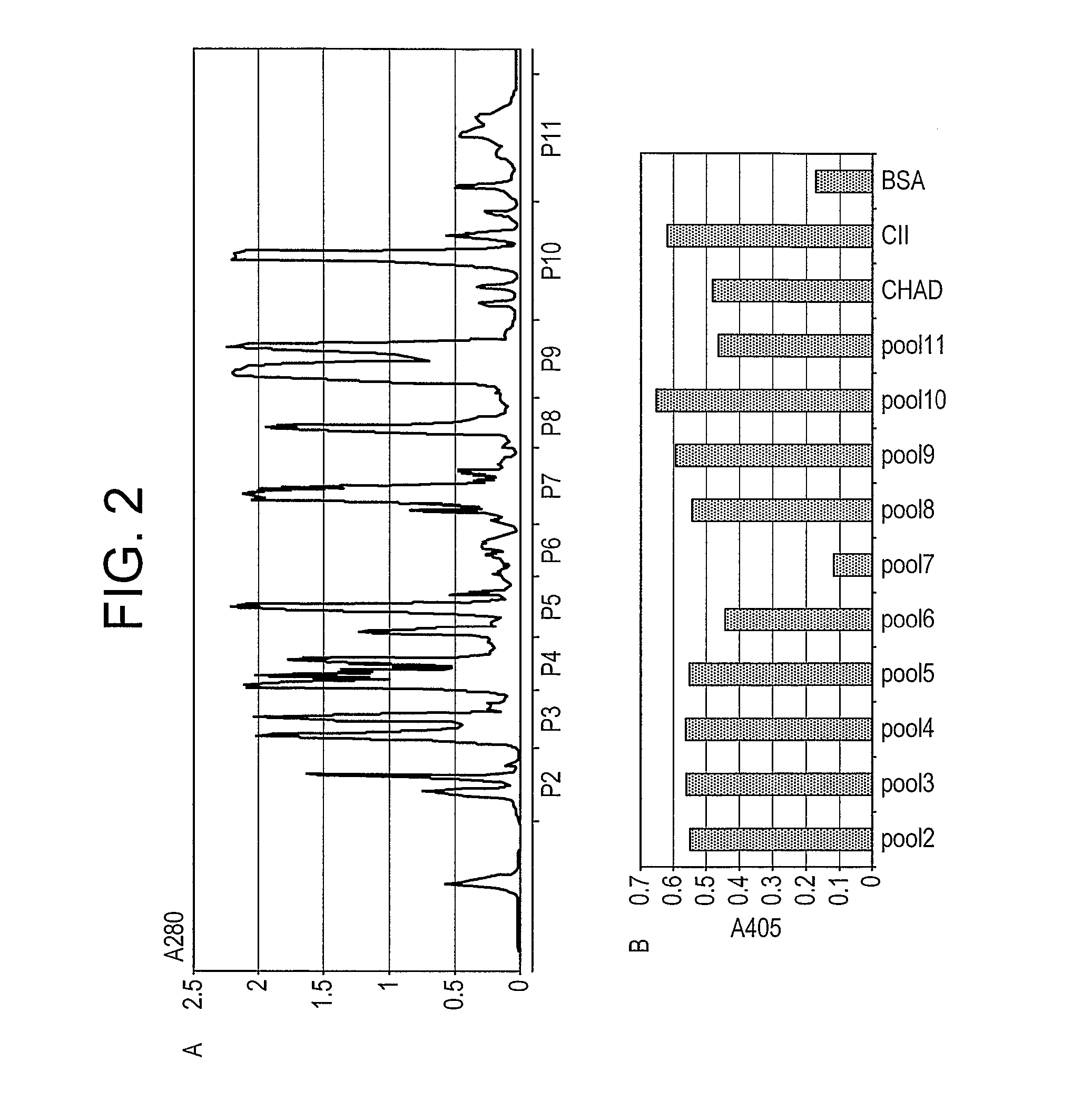
[0131] To obtain evidence that chondroadherin-derived peptides modulate changes of the phenotype of cells involved in a disease associated with tissue changes, we have performed experiments with such cells (chondrocytes, synoviocytes), and in cartilage explants and an arthritis animal model.
[0132] Materials and Methods
Expression and Purification of Recombinant Protein
[0133] Human chondroadherin cDNA was ligated into the pQE8 vector (QIAGEN Inc. Valencia, Calif.) and expressed in M15 E. coli. Overnight cultures (8 ml) of stationary phase bacteria were used to inoculate 400 ml LB-medium (Luria-Bertani) and the cells were allowed to grow for 2.5 hour at 37° C. (OD600 of 0.7-0.9). Protein expression was induced by the addition of 2 mM IPTG (isopropyl-β-D-thiogalactopyranoside), (Sigma Chemicals CO., St Louis, Mo.) and the culture was grown for another 4.5 hour. Bacteria were then coll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| acceleration voltage | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com