Novel Melanocortin Receptor Templates, Peptides, and Use Thereof
a melanocortin receptor and template technology, applied in the field of new chimeric peptides and templates, can solve the problems of affecting the ability of people to interact socially with others, wreak havoc on an individual's mental health, and harmful physical health,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Disulfide Crosslinked or Cyclized Peptides
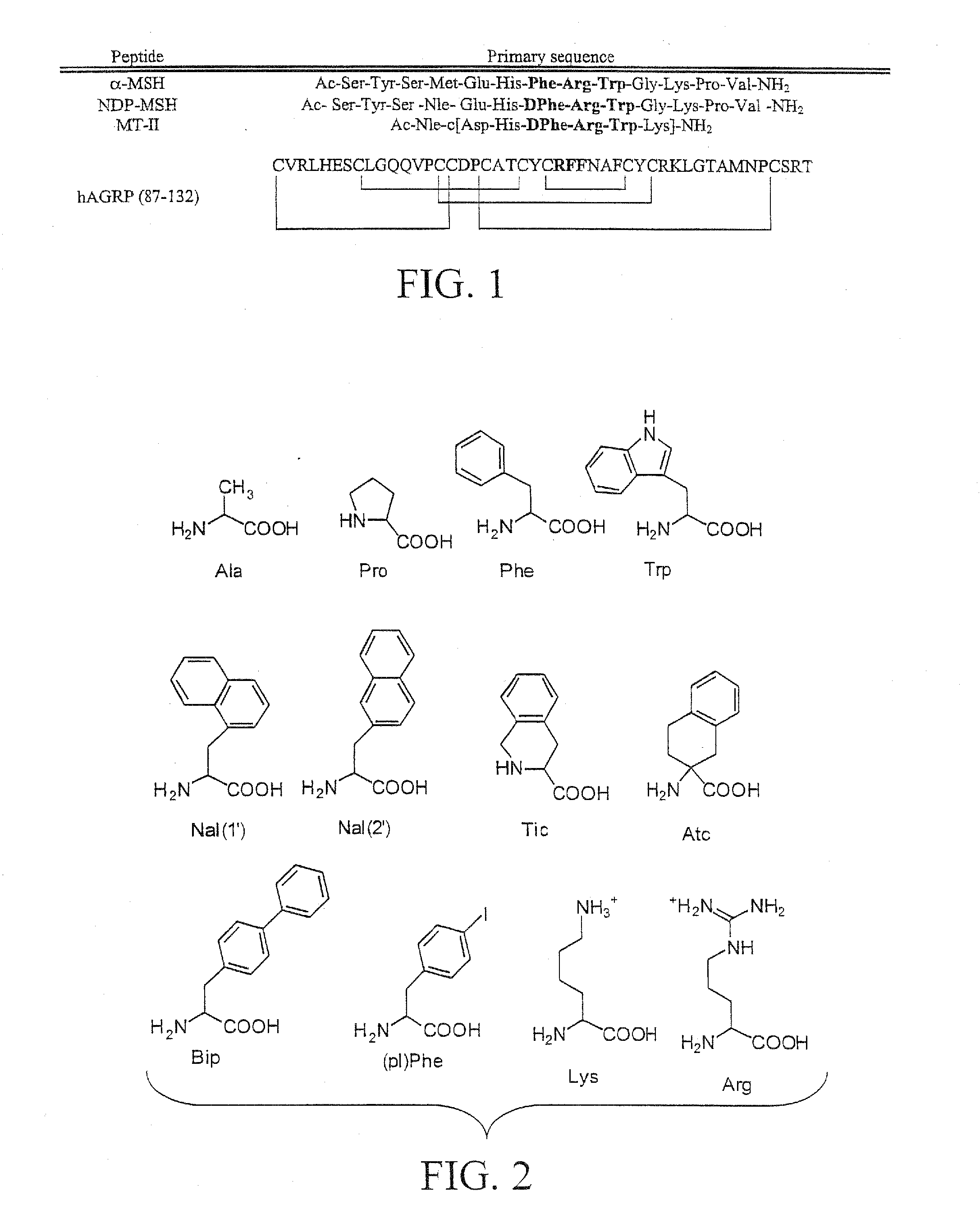
[0068]As understood by the skilled artisan, disulfide cross-linked or cyclized peptides can be synthesized using standard Fmoc methodology as described in Carpino, L. A., and Han, G. Y., “The 9-Fluorenylmethyoxycarbonsy Amino-Protecting Group,”J. Org. Chem., 37:3404-3409 (1972); and Chang, C., and Meienhofer, J., “Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin,”Int. J. Pept. Protein Res., 11:246-249 (1978). Standard Fmoc methodology can be performed on an automated or semi-automated synthesizer (Advance ChemTech 440MOS or LabTech, Louisville, Ky.). The amino acids Fmoc-Ser(tBu), Fmoc-Tyr(tBu), Fmoc-Nle, Fmoc-Glu(OtBu), Fmoc-His(Trt), Fmoc-Arg(Pbf), Fmoc-DPhe, Fmoc-Trp(Boc), Fmoc-Gly, Fmoc-Lys(Boc), Fmoc-Pro, Fmoc-Val, and Fmoc-Phe are all commercially available. All reagents were ACS grade or better.
[0069]Peptides of the p...
example 2
Synthesis of Peptides Containing Cyclic Lactam Bridge
[0074]In accordance with the present invention, peptides containing cyclic lactam bridges can be prepared using standard Boc methodology as described in Merrifield, R. B., “Solid Phase Synthesis. II. The Synthesis of Bradykinin,”J. Am. Chem. Soc., 86:304-305 (1964); and Stewart, J. M., and Young, J. D., Solid Phase Peptide Synthesis, 2nd ed., Pierce Chemical Co., Rockford, Ill. (1964) on an automated synthesizer (Advanced ChemTech 440MOS, Louisville, Ky.). The amino acids Boc-Tyr(2ClBzl), Boc-diaminoprpionic acid [Dpr(Fmoc)], Boc-Asp(OFm), Boc-Arg(Tos), Boc-Phe, Boc-His(Bom), Boc-DPhe, Boc-Trp(CHO), Boc-Asn, and Boc-Ala are commercially available. The peptides were assembled on commercially available pMBHA resin (0.28 meq / g substitution). All reagents were ACS grade or better.
[0075]The synthesis was performed using a commercially available 40 well Teflon reaction block with a course Teflon frit. Approximately 200 mg resin (0.08 mm...
example 3
Assays
[0079]For cell culture and transfection, HEK-293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum and seeded 1 day prior to transfection at 1 to 2×106 cell / 100-mm dish. Melanocortin receptor DNA in the pCDNA3 expression vector (20 μg) were transfected using the calcium phosphate method. Stable receptor populations were generated using G418 selection (1 mg / mL) for subsequent bioassay analysis.
Functional Bioassay
[0080]In the functional bioassay studies, HEK-293 cells stably expressing the mouse MC1, MC3, MC4 and MC5 receptors were transfected with 4 μg CRE / β-galactosidase reporter gene as previously described in Haskell-Luevano, C. et al., “Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors,”J. Med. Chem., 44:2247-2252 (2001); Haskell-Luevano, C. et al., “Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com