Methods and compositions for use in treatment of patients with autoantibody positive disease
a technology for autoantibody positive patients and compositions, applied in the field of methods and compositions for treating patients with autoantibody positive disease, can solve the problems of accumulating symptoms, no one laboratory test that can definitively diagnose lupus, and difficulty in correctly diagnosing lupus patients and patients with other similar diseases, etc., to reduce the frequency and/or quantity of corticosteroids, and reduce the frequency and/or quantity of cortico
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Summary of Results from a Clinical Trial Testing the Use of an Antibody (Belimumab) that Neutralizes Neutrokine-Alpha Protein to Treat Systemic Lupus Erythematosus (SLE)
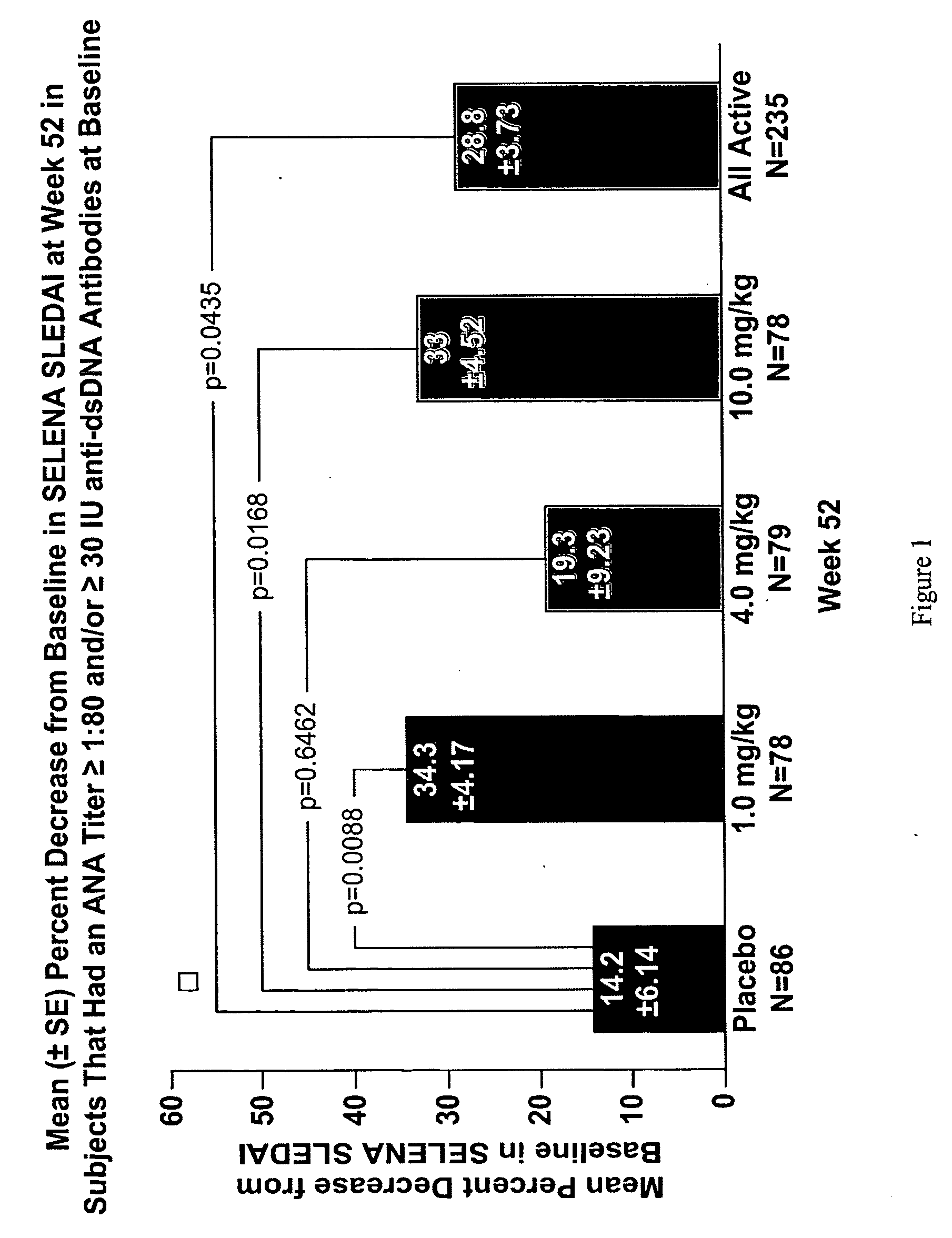
[0563]A prospective, randomized, double-blind, placebo-controlled trial tested belimumab, an antibody that neutralizes Neutrokine-alpha protein, added to standard of care therapy for SLE. 449 subjects with SLE by ACR criteria (Tan et al., Arthritis Rheum. 25:1271-7, (1982); and Hochberg et al., Arthritis Rheum. 40:1725, (1997)), with a history of measurable autoantibodies and SELENA SLEDAI score≧4 at screening were dosed.
[0564]Study agent (1, 4, 10 mg / kg belimumab) or placebo was administered intravenously on days 0, 14, 28 then every 28 days over 52 weeks. Subjects who completed the 52-week treatment period were given the option to continue the study for a 24-week extension period. Belimumab was formulated in 10 mM sodium citrate, 1.9% glycine, 0.5% sucrose, 0.01% (w / v) polysorbate 80, pH 6.5 (±0.3). Subjects receiv...
example 2
Scoring Proteinuria for SELENA SLEDAI
[0571]Kidney malfunction is often associated with Systemic lupus erythematosus. One of skill in the art would be aware of a variety of standard measures that can be used to assess kidney function, for example, progression to end-stage renal disease, sustained doubling of serum creatinine, creatinine clearance, iothalamate clearance, protein concentration in a single urine sample and protein concentration in a 24-hour urine sample.
[0572]Changes in proteinuria calculated from “24 hour urine samples” is one of the categories scored in the SELENA SLEDAI. Proteinuria measurements may be performed by any method known in the art. In a specific embodiment, a single urine specimen is collected and the amount of protein and / or creatinine clearance is measured, see, for example, Lemann, et al., Clin Chem., 33:297-9, 1987. and Schwab, et al., Arch Intern Med., May; 147(5):943-4, 1987. In a specific embodiment, urine is collected over 24 hours and the amount ...
example 3
Summary of Results from a Clinical Trial Testing the Use of an Antibody (Belimumab) that Neutralizes Neutrokine-Alpha Protein to Treat Rheumatoid Arthritis (RA)
[0576]A Phase 2, multi-center, randomized, double-blind, placebo-controlled study was performed in subjects with RA. Subjects were randomized into 4 treatment groups (placebo, 1 mg / kg, 4 mg / kg and 10 mg / kg). Belimumab or placebo was administered at doses of 1, 4 and 10 mg / kg on Days 0, 14 and 28 and every 28 days thereafter for 24 weeks, followed by an optional 24-week extension period. Belimumab was formulated in 10 mM sodium citrate, 1.9% glycine, 0.5% sucrose, 0.01% (w / v) polysorbate 80, pH 6.5 (±0.3). Subjects receiving placebo dose received the formulation (10 mM sodium citrate, 1.9% glycine, 0.5% sucrose, 0.01% (w / v) polysorbate 80, pH 6.5 (±0.3) without belimumab. A total of 283 subjects participated in the study. Belimumab was administered to 214 subjects at doses of 1, 4, or 10 mg / kg during the 24-week treatment phas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com