Combinatorial therapy
a conjugate therapy and vegf technology, applied in the field of conjugate therapy, can solve the problems that many patients treated with vegf antagonists eventually succumb to their disease, and achieve the effects of reducing or preventing antibody dependent cellular cytotoxicity (adcc) or complement dependent cytotoxicity (cdc) activity, improving effector function, and reducing or preventing antibody dependent cellular cytotoxicity (adcc) or cdc activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
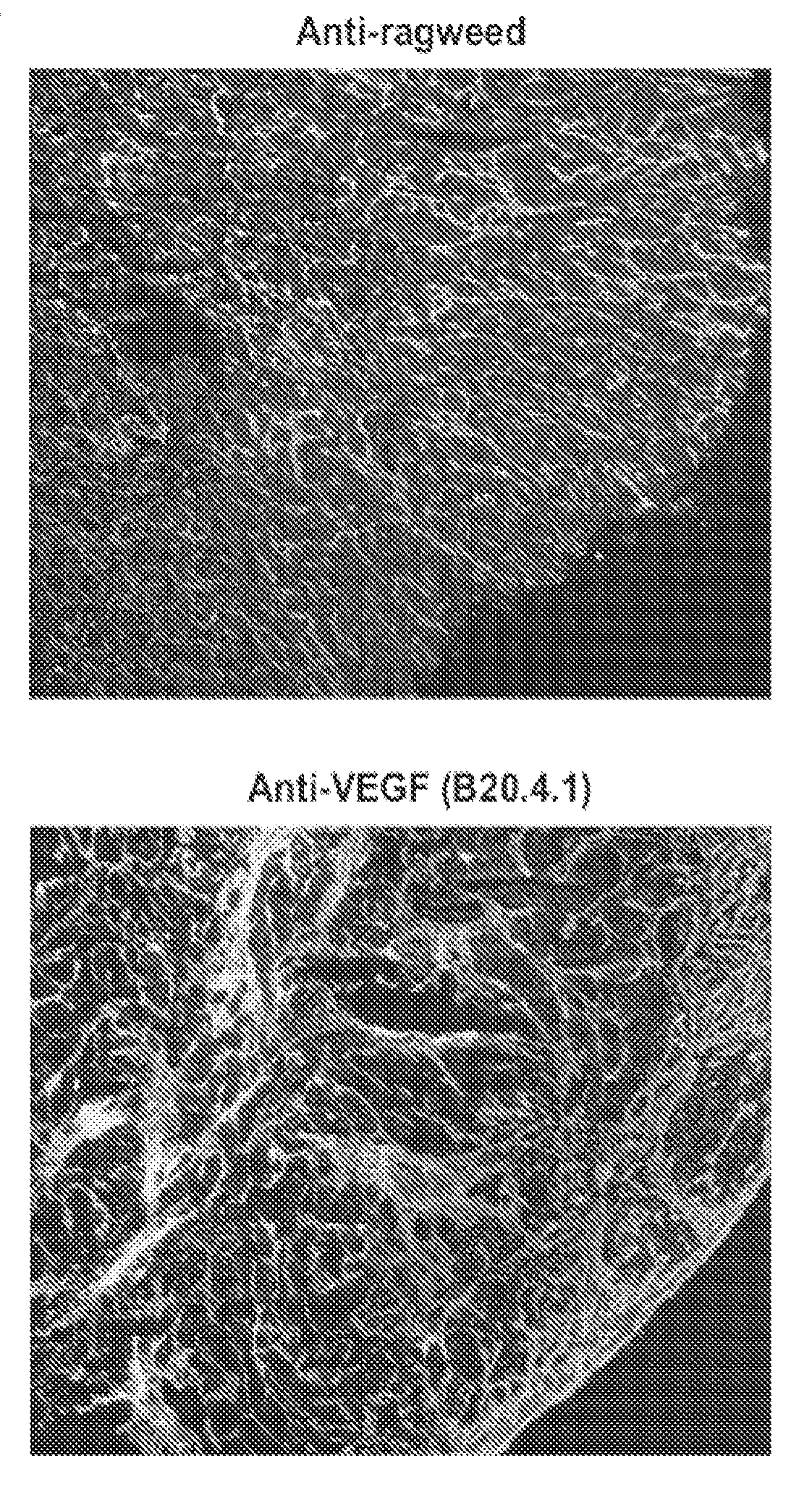
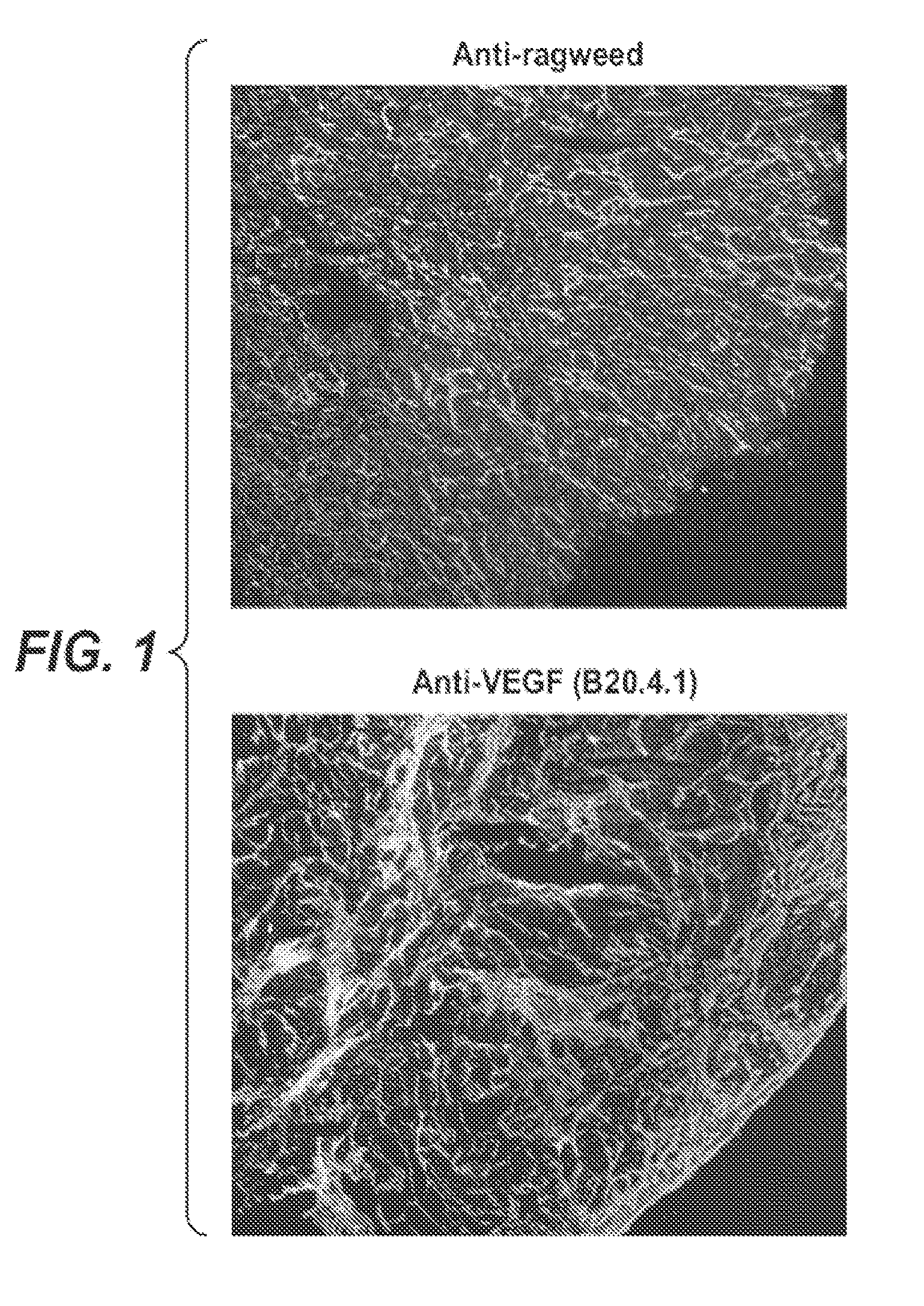
Recruitment of Alpha5Beta1-Expressing Stromal Cells After Anti-VEGF Therapy
[0215]Sections of HT-29 human colorectal carcinoma xenografts that had been treated with anti-VEGF antibody B20-4.1 monotherapy in athymic mice were stained for anti-alpha5beta1 expression. Compared to a control group treated with a control antibody (anti-ragweed antibody) in this study, B20-4.1 monotherapy yielded a median time to endpoint (TTE) that corresponded to little or no activity. The tumors had been measured twice weekly for the duration of 58 days. Animals were euthanized when their tumors reached the endpoint volume of 1000 mm3 or on Day 58, whichever came first, and the (TTE) was calculated for each mouse. Treatment outcome had been determined from percent tumor growth delay (% TGD), defined as the percent increase in median TTE of treated versus control mice, with differences deemed significant at 0.01≦P≦0.05, and highly significant at P<0.01 using Logrank analysis. The median TTE value of the c...
example 2
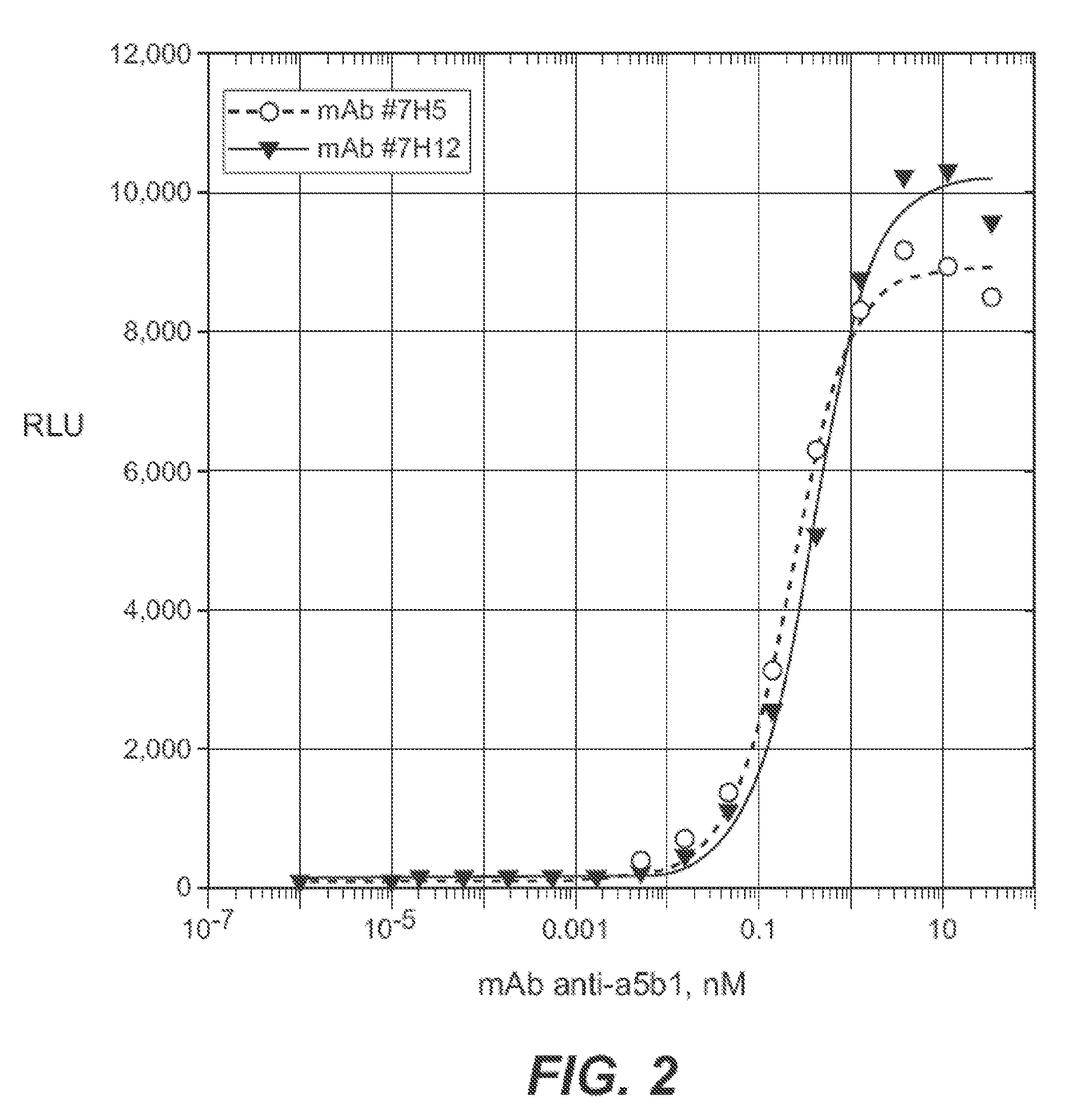
Anti-Alpha5Beta1 Antibodies
[0217]Mice were injected with purified human alpha5beta1 (Chemicon CC1027). Plasmacytoma cells expressing anti-alpha5beta1 antibodies were isolated and transformed into hybridoma cell lines. Two hybridoma cell lines designated 7H5.4.2.8 and 7H12.5.1.4 were deposited with the ATCC. See above. The antibody produced from the 7H5.4.2.8 hybridoma is a mIgG2a Kappa antibody (also referred to herein as the “7H5 antibody”). The antibody produced from the 7H12.5.1.4 hybridoma is a mIgG2b Kappa antibody (also referred to herein as the “7H12 antibody”).
example 3
HUVEC Direct Binding Assay
[0218]Tissue cultures containing growing human umbilical vein endothelial cells (HUVEC) were washed twice with PBS. The cells were detached from the culture flask with 3-4 ml of a 5 mM EDTA / PBS solution. Fresh culture media was added to the cells and mixed. An aliquot of the cells in the mixture was counted. Cells were centrifuged and washed with wash buffer (50 mM Tris, 150 mM NaCl, pH7.5) one time. The cell concentration was adjusted so that the cells could be seeded at 25 ul per well onto 96-well MSD high binding plates at 25,000 cells / well or 4,000 cells / well on a 384 well plate (Cat #L11XB-1 or #L11XB-2, respectively, Meso Scale Diagnostics, LLC). The cells were incubated 1 hour at room temperature on the plate to allow capture. To block the well, 25 ul of stock buffer (30% fetal bovine serum (FBS) in TBS (50 mM Tris, 150 mM NaCl)+1 mM CaCl2 / 1 mM MgCl2, pH7.5) was added to the well and incubated at room temperature to for 30 minutes to 1 hour.
[0219]Ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com