Compound comprising an autoantigenic peptide and a carrier with a mhc binding motif
a technology of mhc binding and peptide, which is applied in the direction of peptide/protein ingredients, fusions for specific cell targeting, antibody medical ingredients, etc., can solve the problems of inability to cure and often worse symptoms, and achieve the effect of reducing disease and/or eliminating inflammatory joint diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of the MHC Class II Aq Constructs
[0044]The cDNAs for Aalphaq and Abetaq were amplified from a first strand cDNA reaction (first strand cDNA, Pharmacia, Piscataway, N.J.). The cDNAs were further modified to include cloning sites immediately upstream of the start codon, and the 3′ end from the transmembrane domain and downstream was replaced by an inframe cloning site. Next, DNA for the leucine zipper (13) domain from Jun including a 3′ end coding for 6 histidines was cloned in frame with the beta-chain cDNA. The DNA for the leucine zipper domain from Fos was added to the alpha chain construct. The resulting constructs were cloned separately into pMTAL (Invitrogen, La Jolla, Calif.) or pRmHa-3 (14) to allow for heavy metal-induced expression in insect cells. pMTAL contains the resistance gene for hygromycin. Where pRmHa-3 was used a Copia promoter-driven hygromycin gene was used as selection marker.
example 2
Transfection, Expression and Purification of Soluble Aq
[0045]The linearised Aq alpha-chain and Aq beta-chain constructs were co-transfected at equimolar ratios into Drosophila melanogaster SL2 cells (ATCC, CRL-1963) using calcium phosphate transfection. Stable transfectants were derived by hygromycin selection and kept under selection in Schneider's Drosophila medium (Gibco™, Paisley, Scotland, UK) containing 100 μg / ml of hygromycin B (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Large-scale cell cultures were prepared in Fernbach bottles using a magnetic stirrer. For expression of soluble Aq, transfected cells were grown in serum-free Insect express complete medium (PAA Laboratories GmbH, Linz, Austria) at 25° C., induced with 0.7 mM CuSO4 for three days, and the supernatants were clarified by centrifugation and filtration. The SL2 cells produced ˜2-3 mg of recombinant protein per liter of culture. The expressed soluble Aq molecules were purified from the clarified media using ...
example 3
ELISA, SDS-PAGE and Western blot analyses of Aq
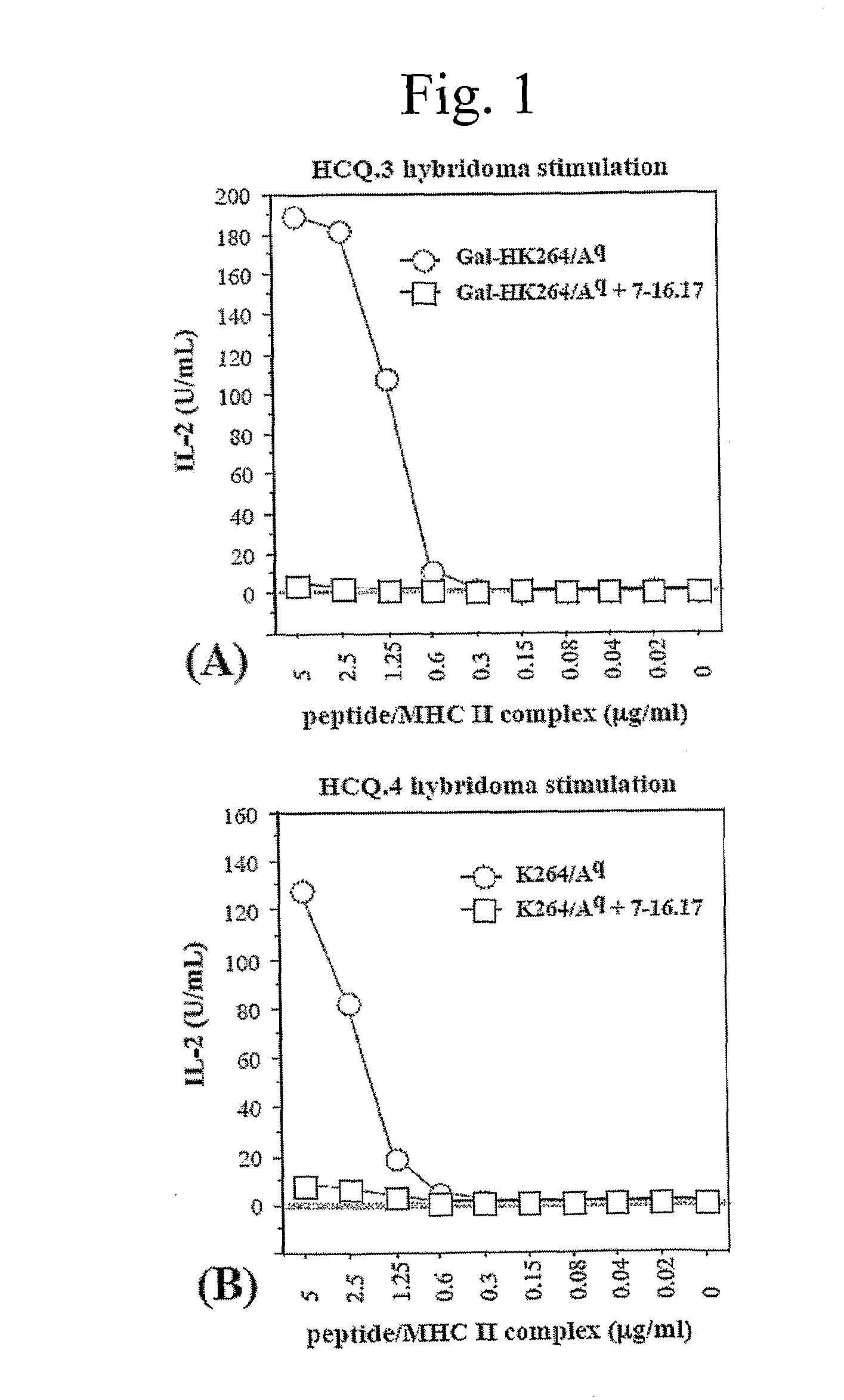
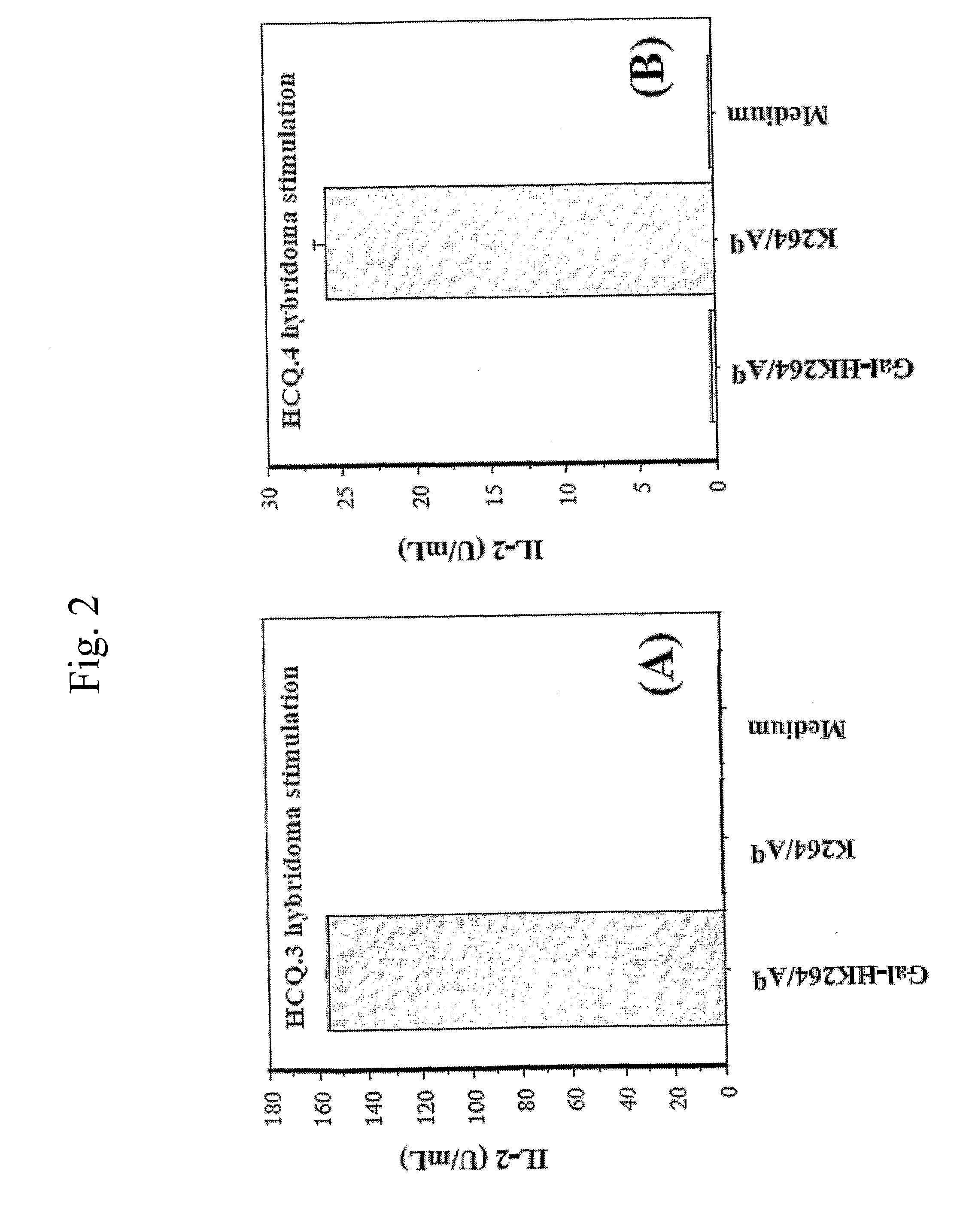
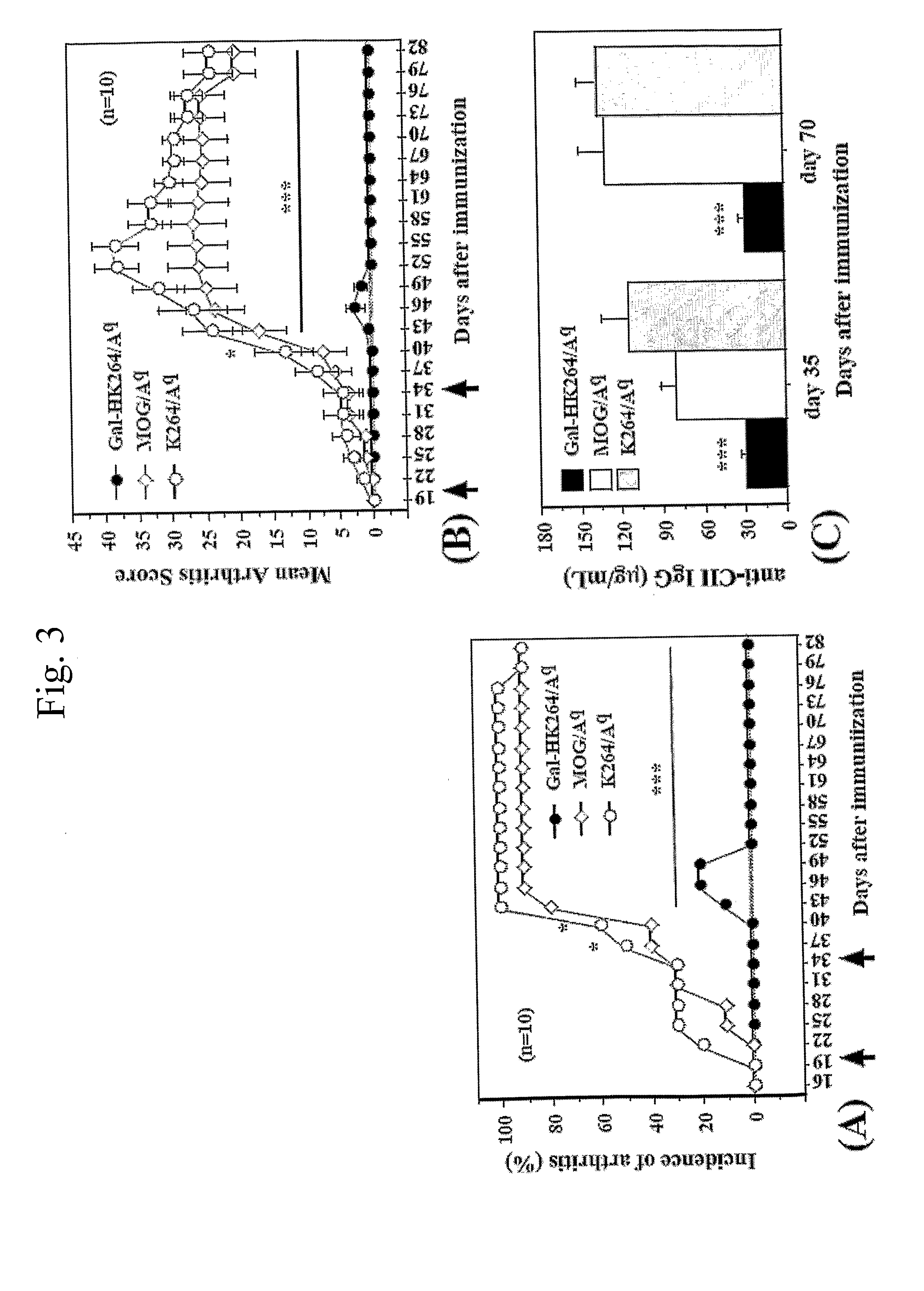
[0046]The alpha- and beta-chains of the purified Aq protein were detected by sandwich ELISA, using Y3P mAb (specific for the native alpha-chain) as capturing antibodies and biotinylated 7-16.17 (BD PharMingen, Los Angeles, Calif.) mAb (specific for the beta-chain) as detecting antibodies. Flat-bottom 96-well plates (Nunc, Roskilde, Denmark) were coated with 2.5 μg / mL Y3P and incubated overnight at 4° C. The plates were then washed with PBS, blocked with 1% BSA (Sigma, St Louis, Mo.) in PBS for 1 h, washed again, and incubated for 2 h with 50 μL from the protein fractions at room temperature. Plates were washed again, followed by addition of 1 μg / mL biotinylated 7-16.17 for 1 h. After washing, the biotin-labeled antibody was detected by europium-labeled streptavidin using the DELFIA system (Wallac, Turku, Finland).
[0047]Protein purity was assessed by SDS-PAGE. Samples were electrophoresed in 4-20% polyacrylamide gradient ready mini-gels...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com