Therapy for the treatment of disease
a technology for treating diseases and diseases, applied in drug compositions, animal repellents, extracellular fluid disorders, etc., can solve the problems of lack of specificity, oab symptoms are generally underreported by patients, and healthcare professionals are not able to treat them effectively, so as to relieve constipation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Combination of an OAB Drug and a Salivary Gland Stimulant for the Treatment of Individual with Overactive Bladder
[0114]An individual with overactive bladder is identified. The individual is given 5 mg of oxybutynin two to four times a day in addition to 5 mg of pilocarpine two or three times a day. If the individual continues to complain about dry mouth, the dose of pilocarpine is increased to 10 mg two or three times a day. The dose can be increased up to 20 mg, or 50 mg, if needed. Each dose of oxybutynin can be increased to 10, 15, 20, or 30 mg.
example 2
Combination of an OAB Drug and a Tegaserod for the Treatment of Individual with Overactive Bladder
[0115]An individual with overactive bladder is identified. The individual is given 5 mg of oxybutynin two to four times a day in addition to 2 mg of tegaserod twice a day. If the individual continues to complain about constipation, the dose of tegaserod is increased to 6 mg twice a day. The dose can be increased up to 12 mg, 20 mg, or 50 mg, if needed. The dose of oxybutynin can be increased to 10, 15, 20, or 30 mg.
example 3
Clinical Study Protocol Synopsis
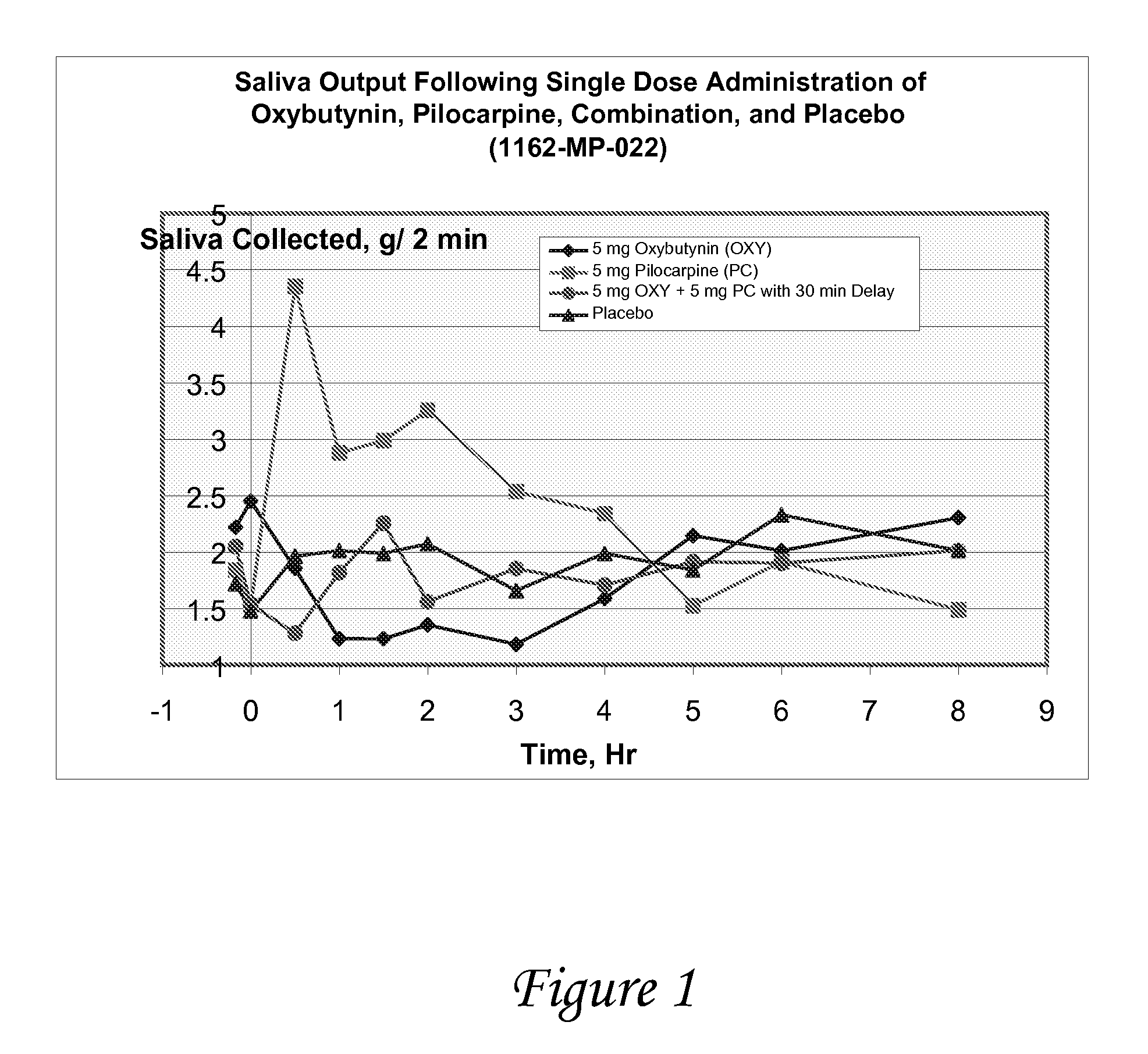
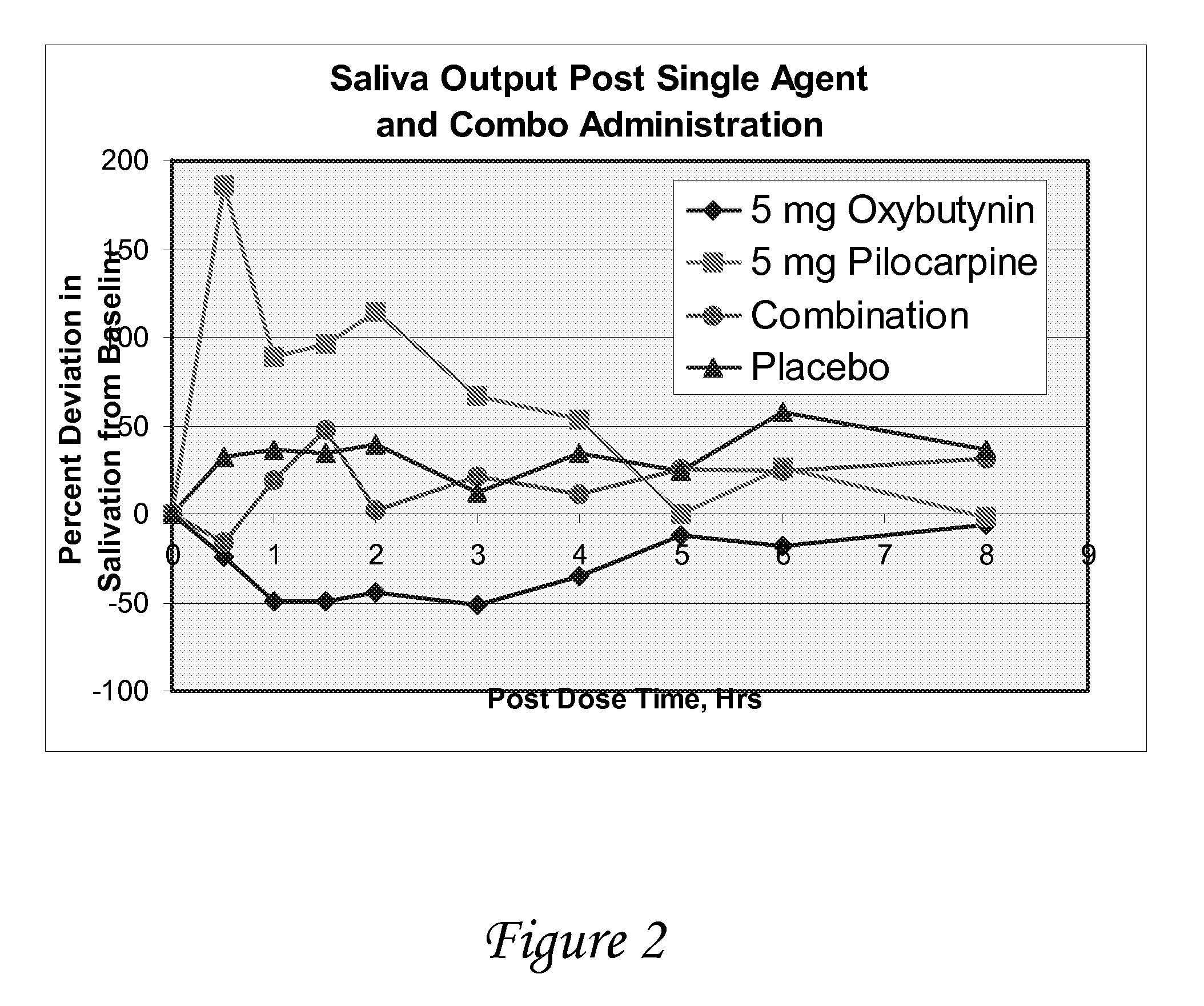
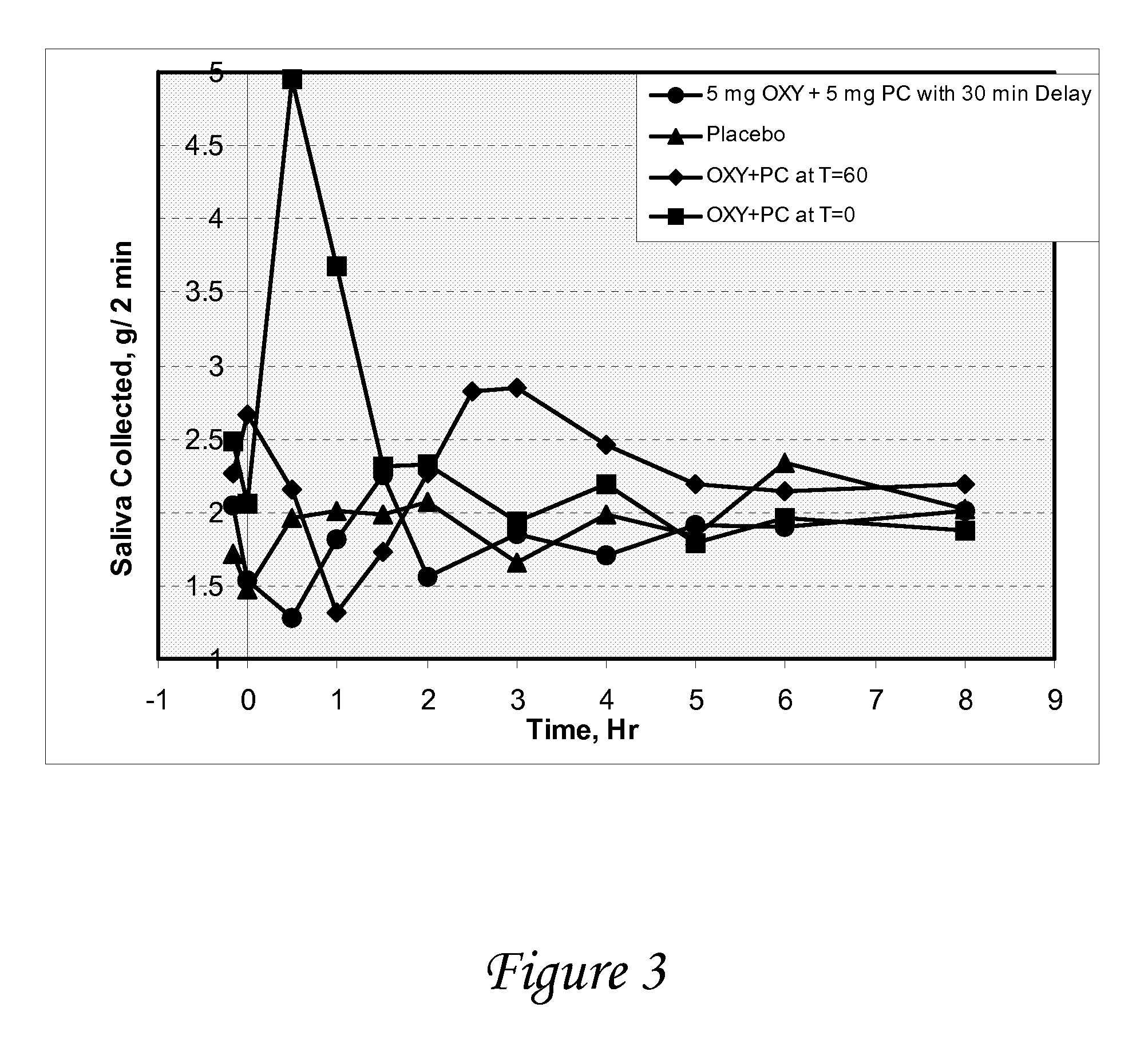
[0116]A study is conducted to evaluate the effect of oxybutynin and pilocarpine, alone and in combination versus placebo on salivary output in healthy volunteers. The objectives of the study are to determine salivary flow and degree of dry mouth after oral administration of oxybutynin and pilocarpine, alone and in combination, vs. placebo, and to determine the effect of oxybutynin and pilocarpine, alone and in combination, on urine volume / void and vital signs.
[0117]At each treatment period, following an overnight fast, subjects enter the clinic and after baseline measurements have been made, they are randomized to one of four medications
[0118]Oxybutynin (5 mg) followed 30 minutes later by placebo
[0119]Pilocarpine (5 mg) followed 30 minutes later by placebo
[0120]Placebo followed 30 minutes later by placebo
[0121]Oxybutynin (5 mg) followed 30 minutes by pilocarpine (5 mg)
[0122]The following measurements are made just prior to and at frequent intervals fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| nighttime urinary frequency | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com