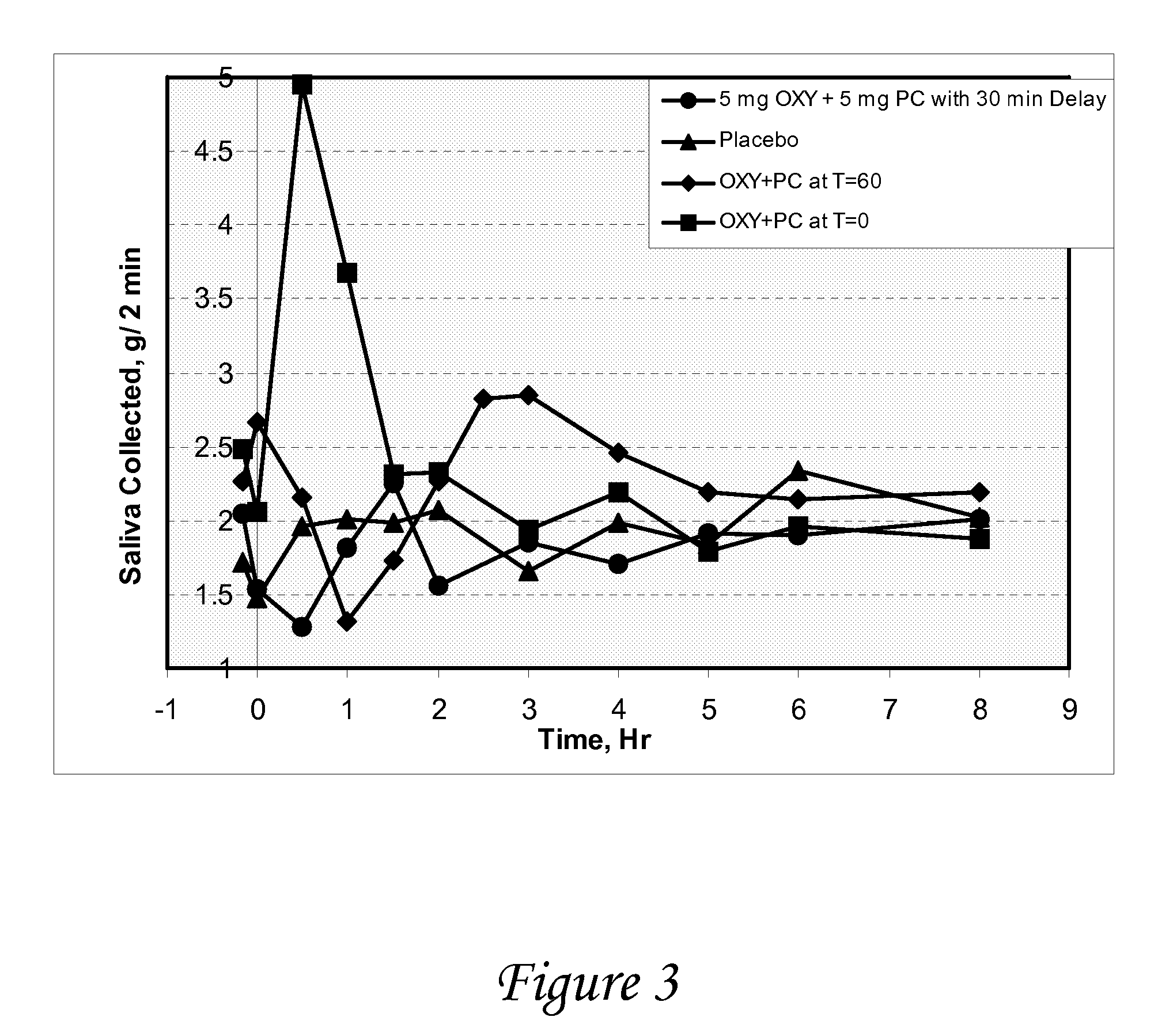
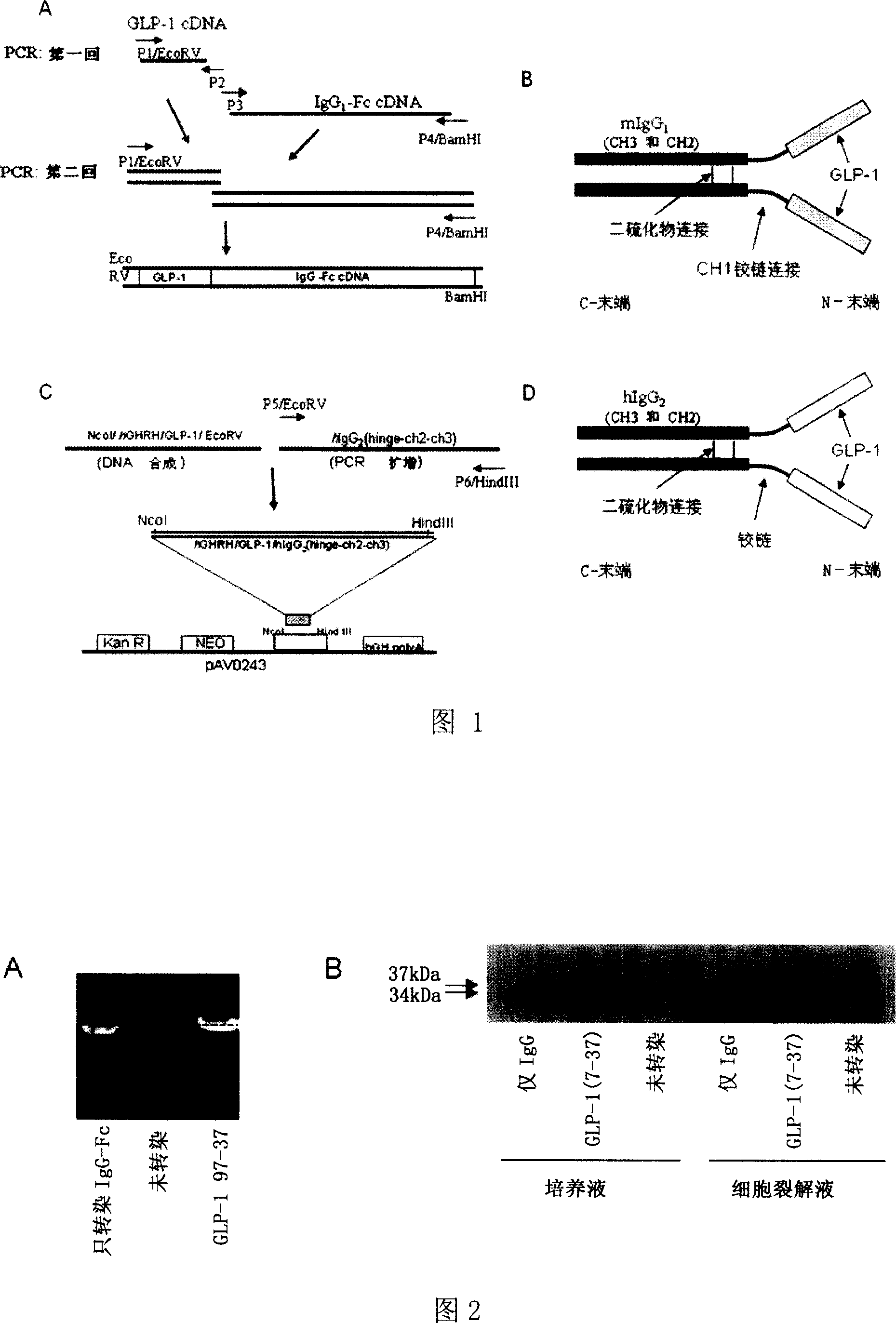
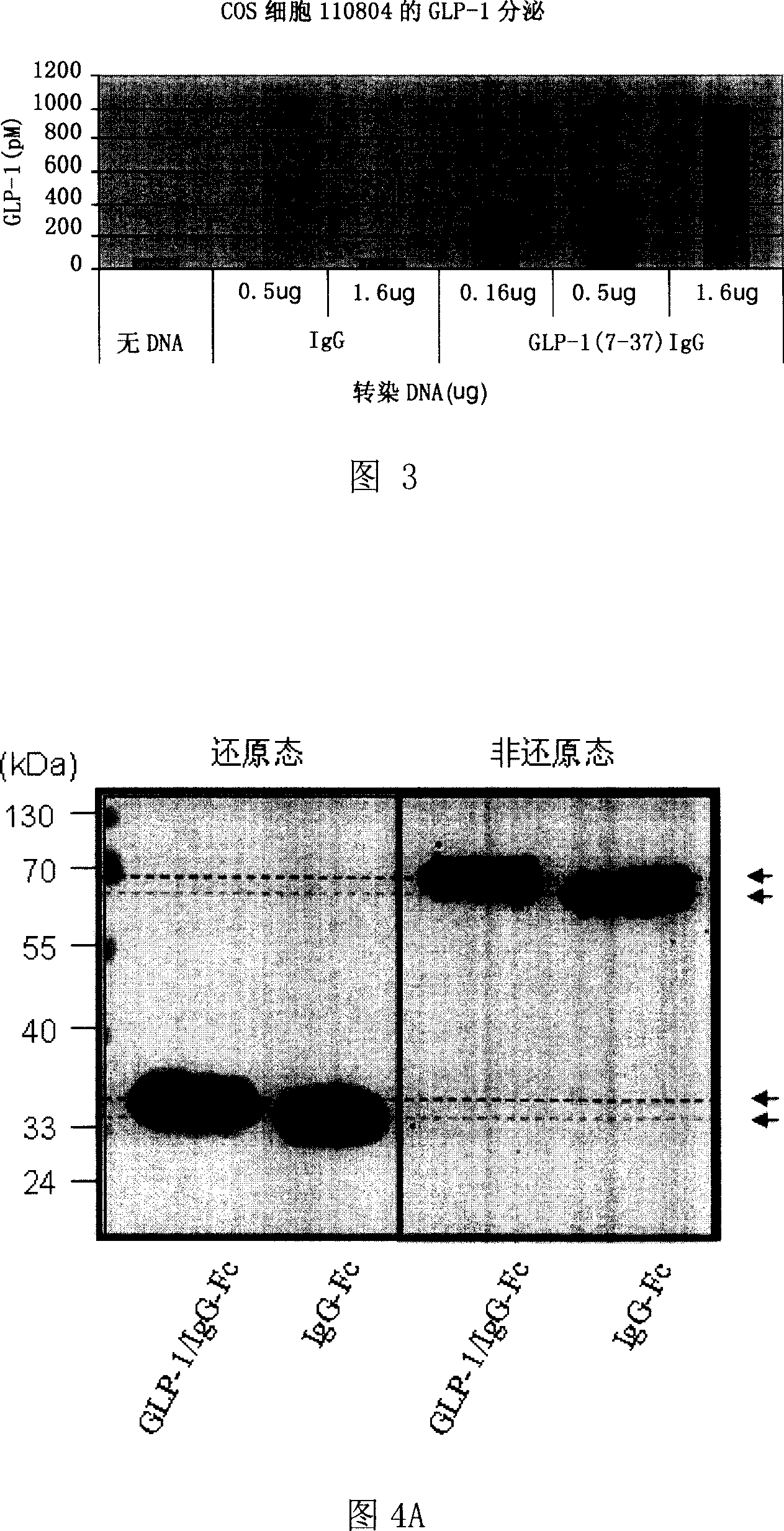
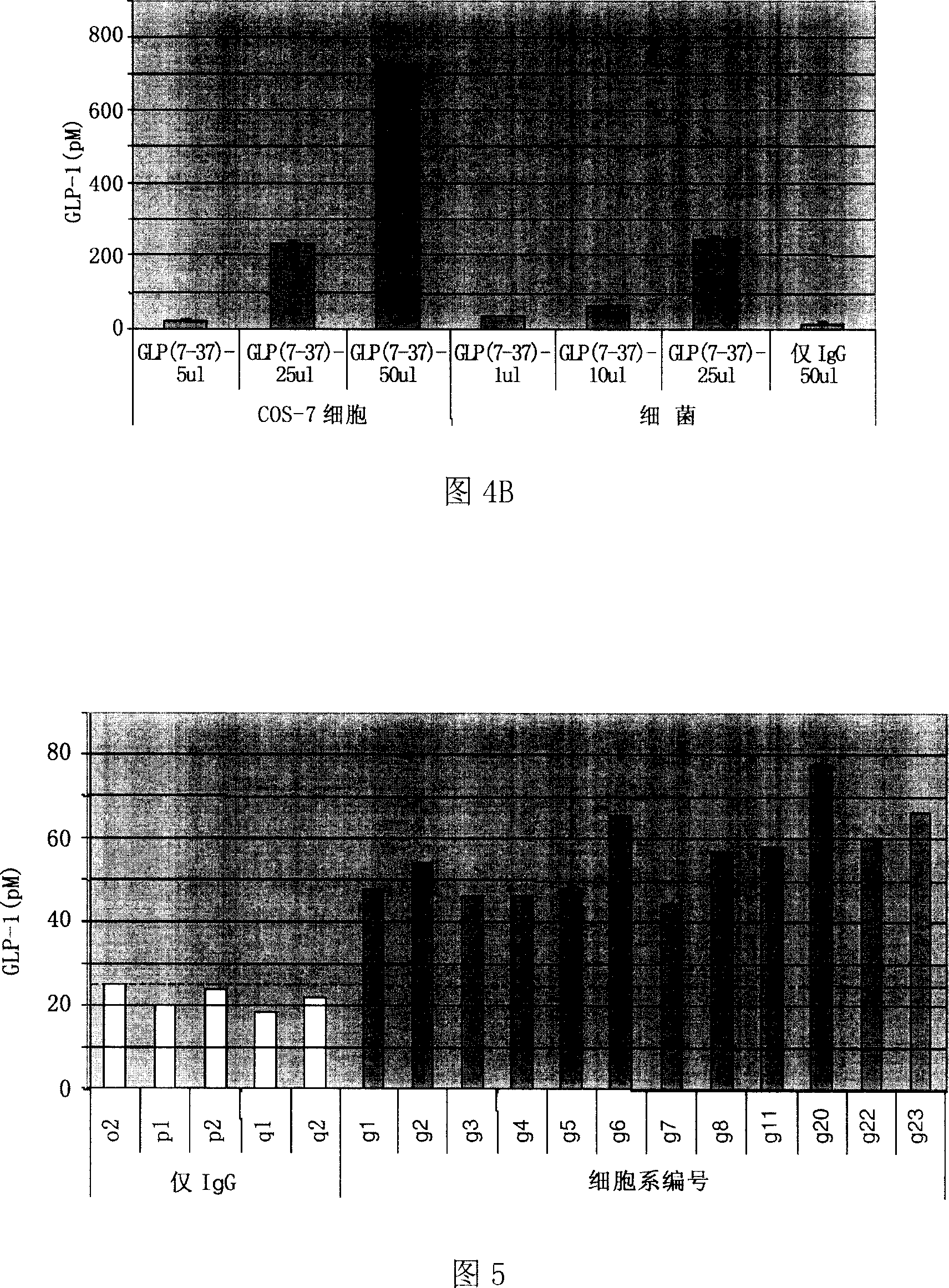
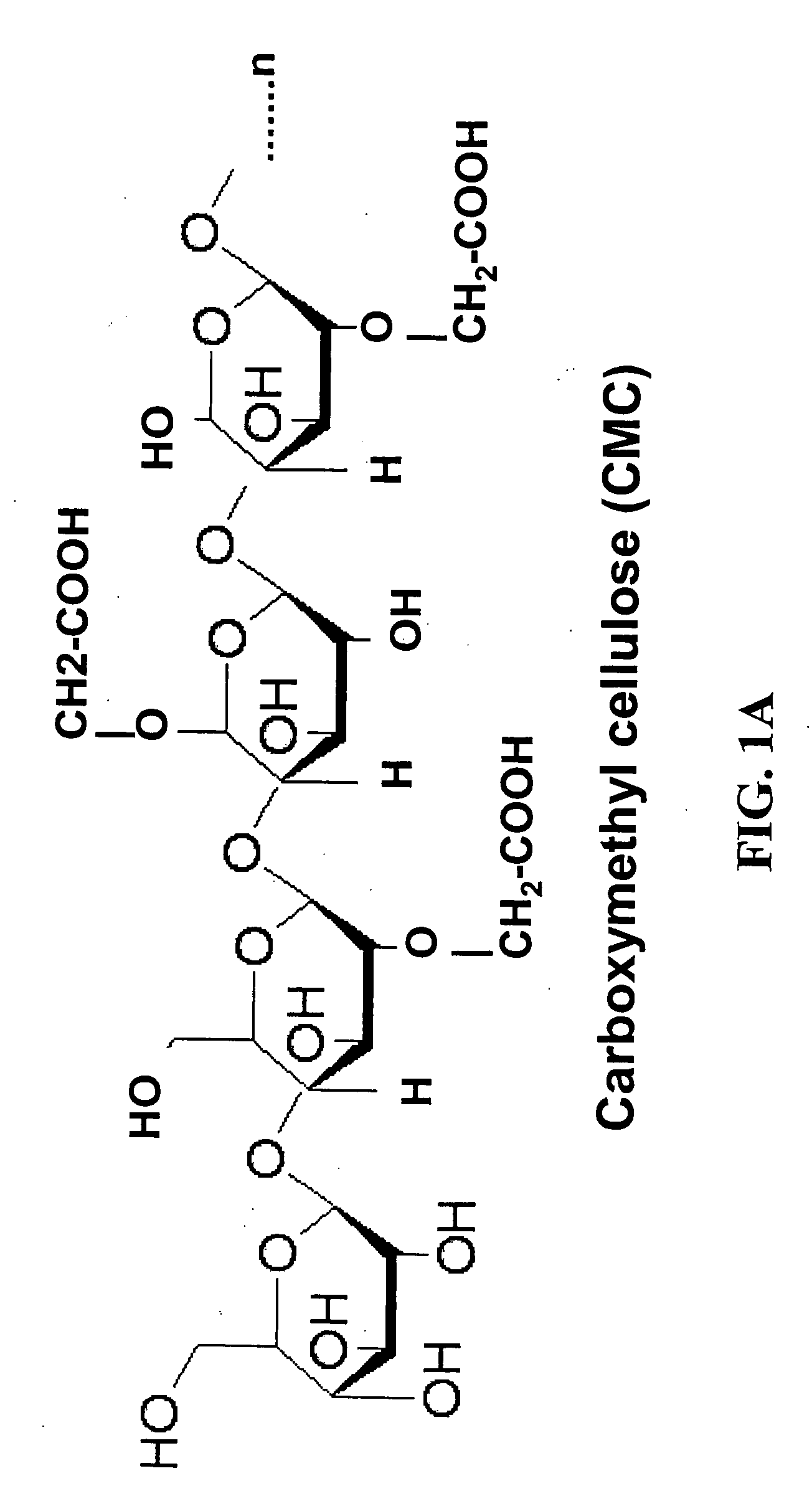
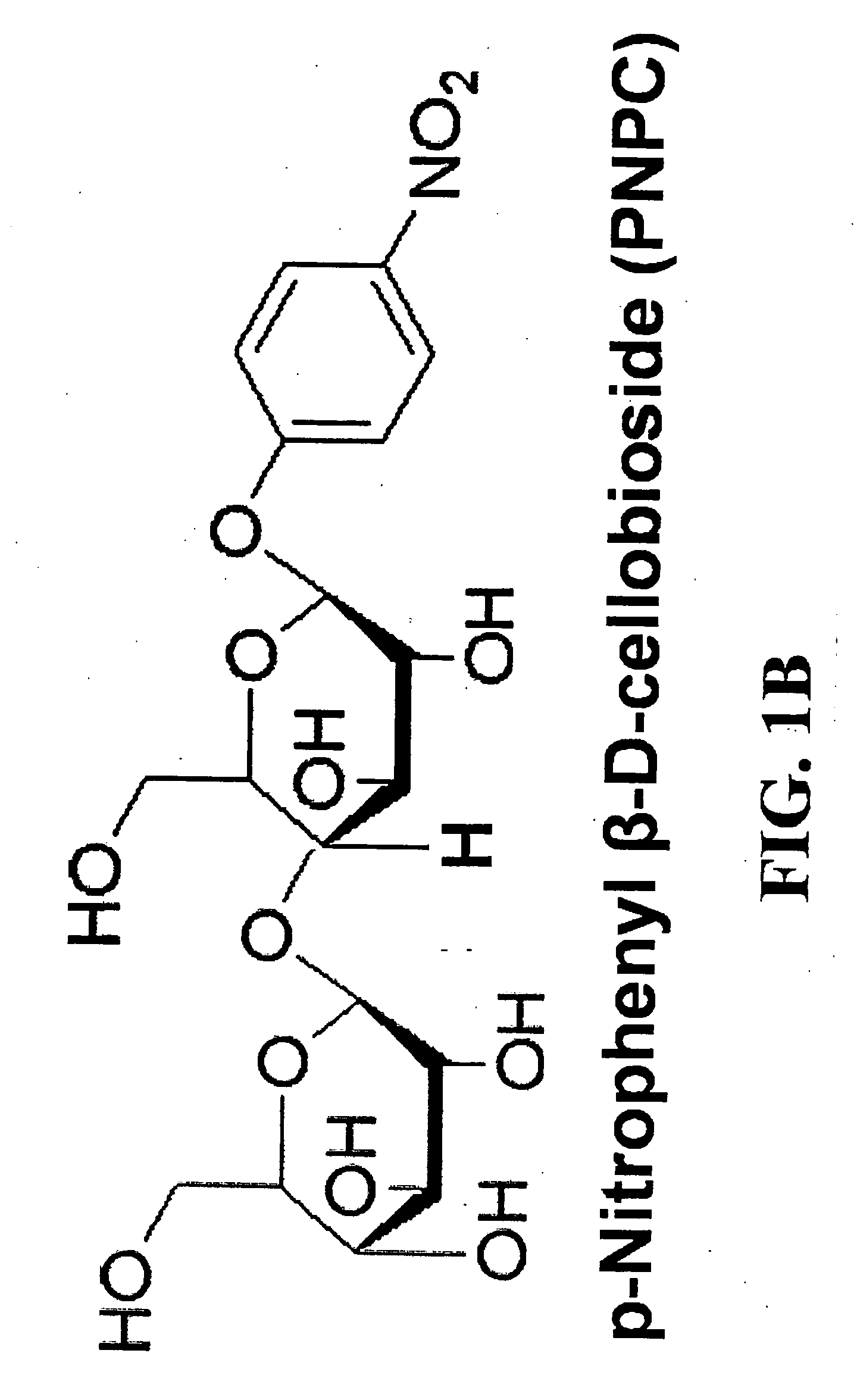
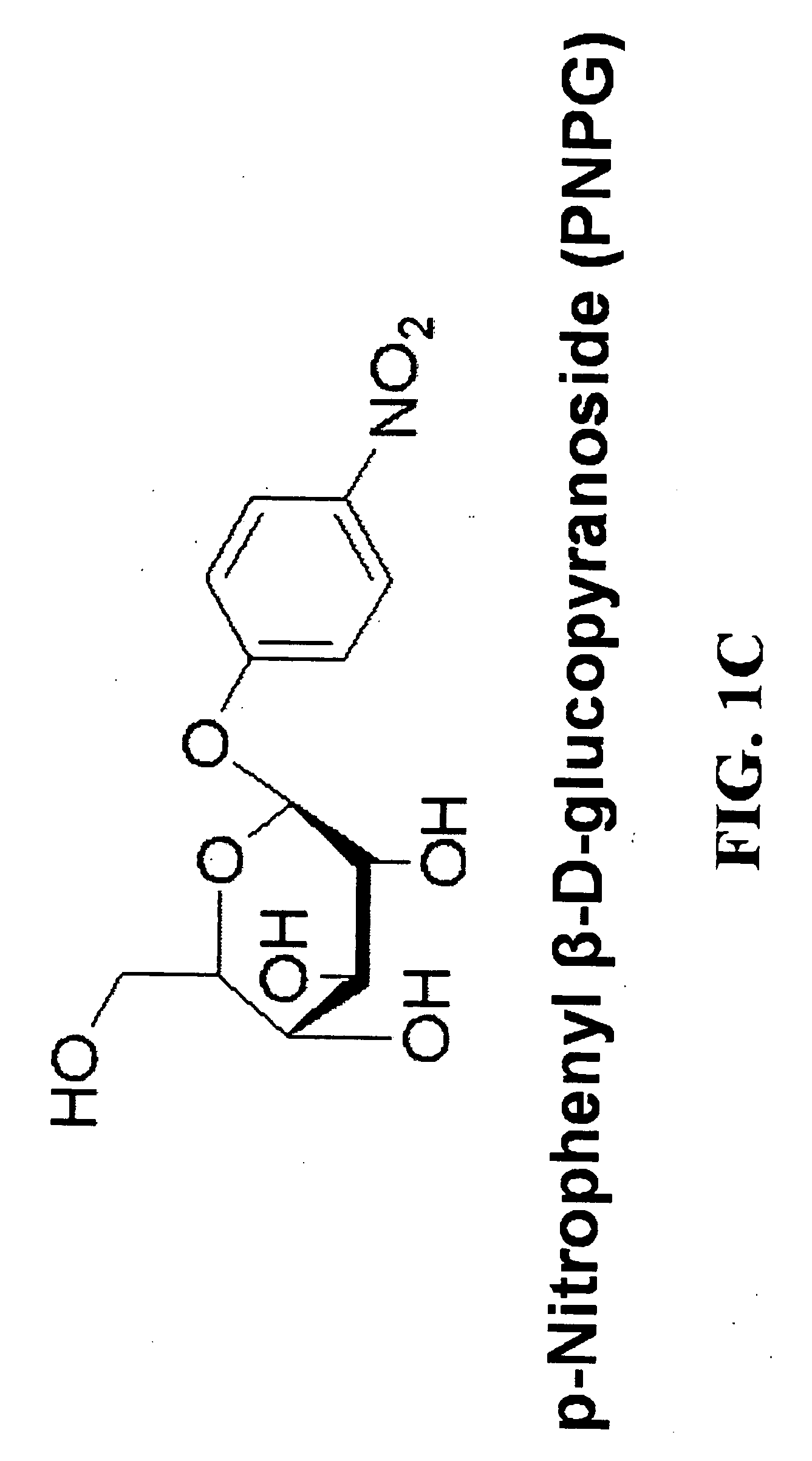
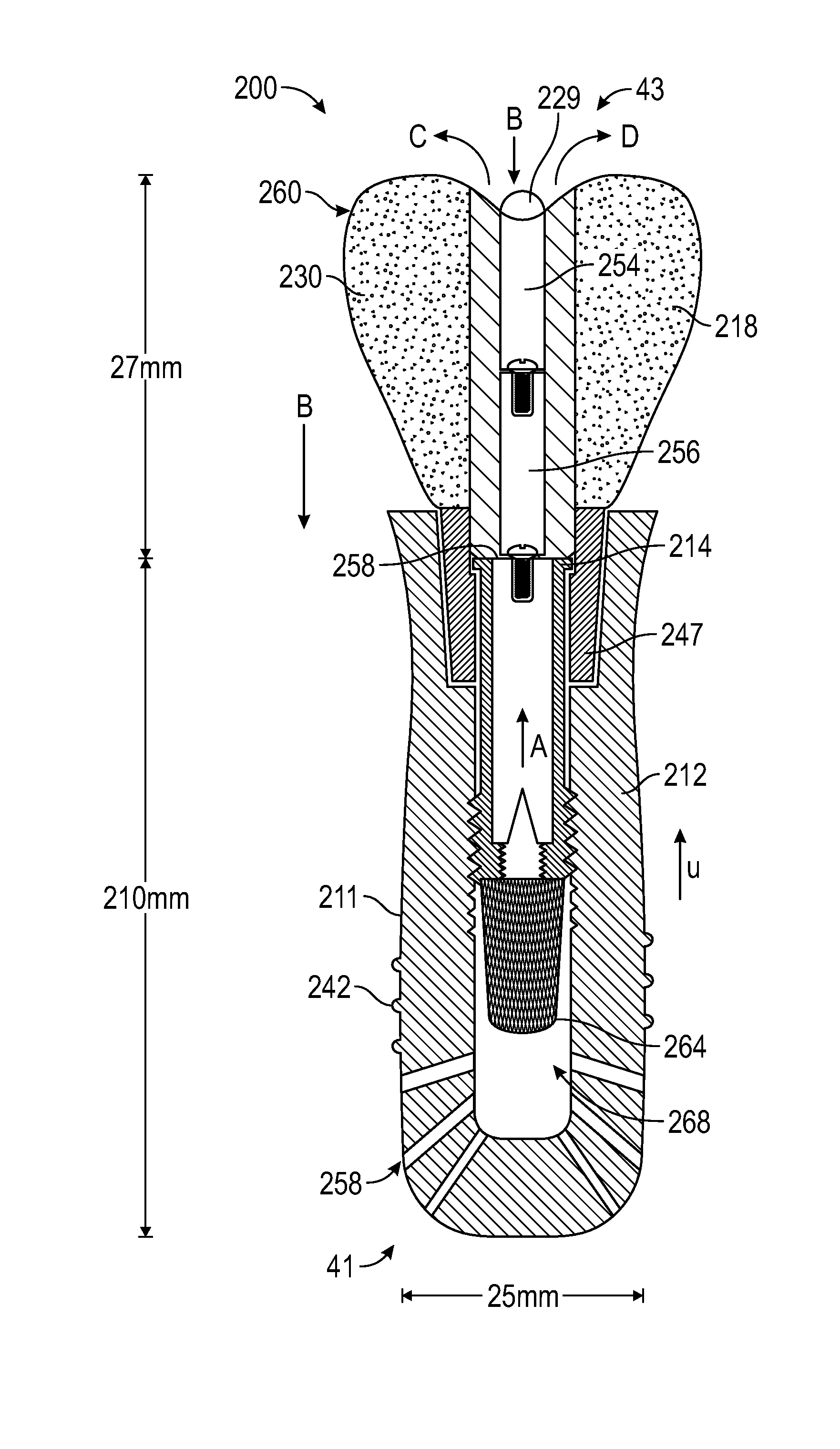
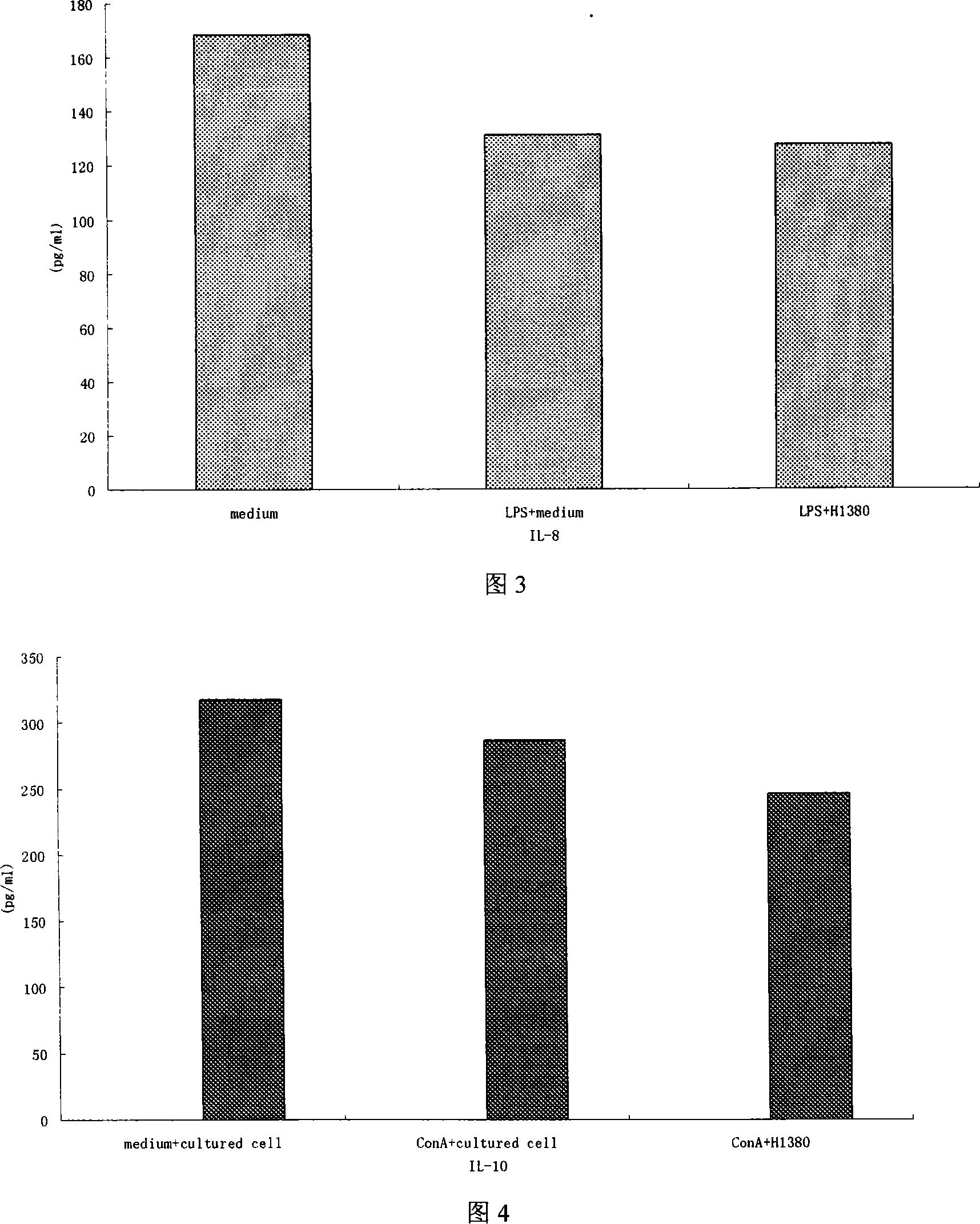
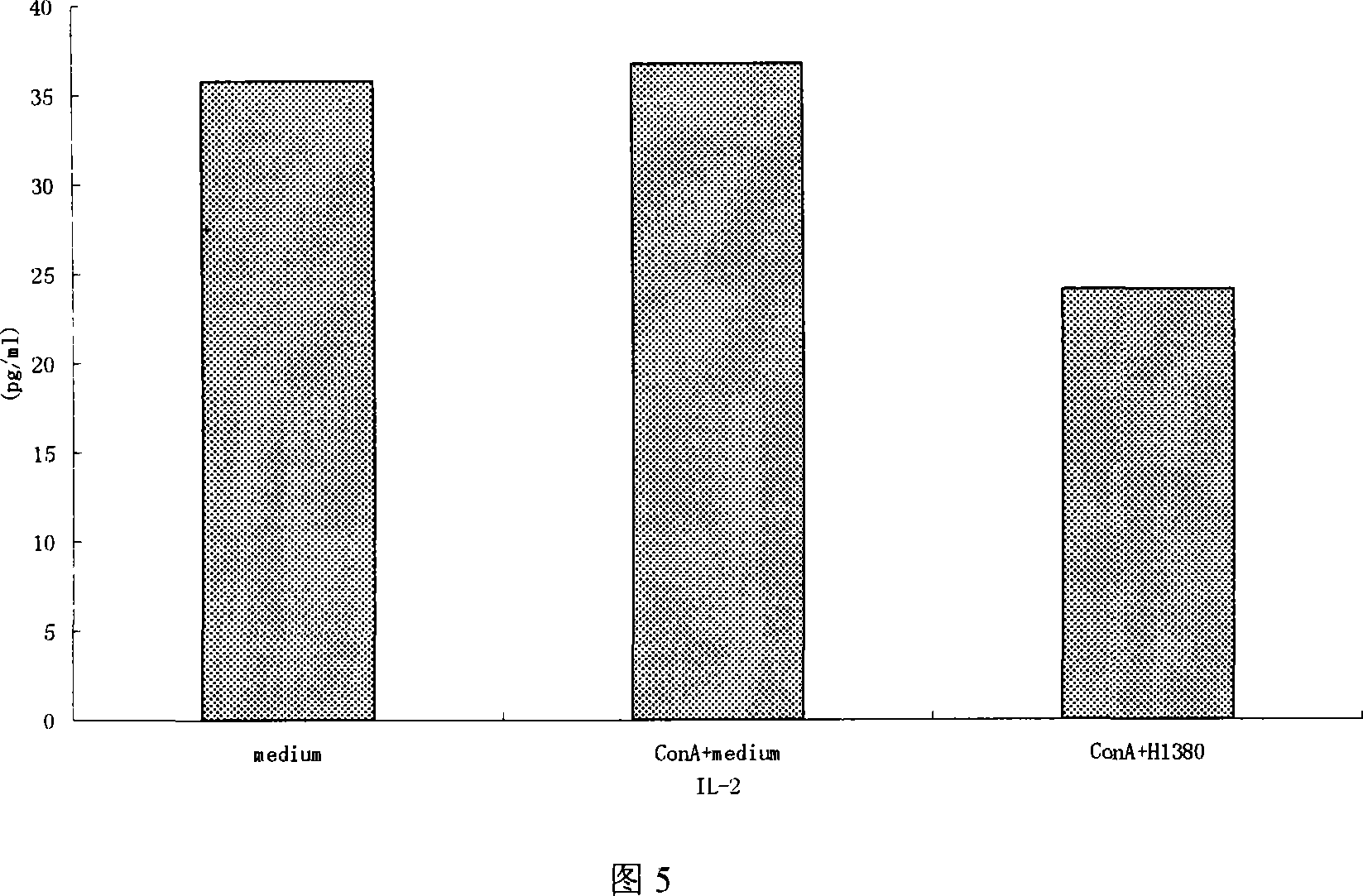
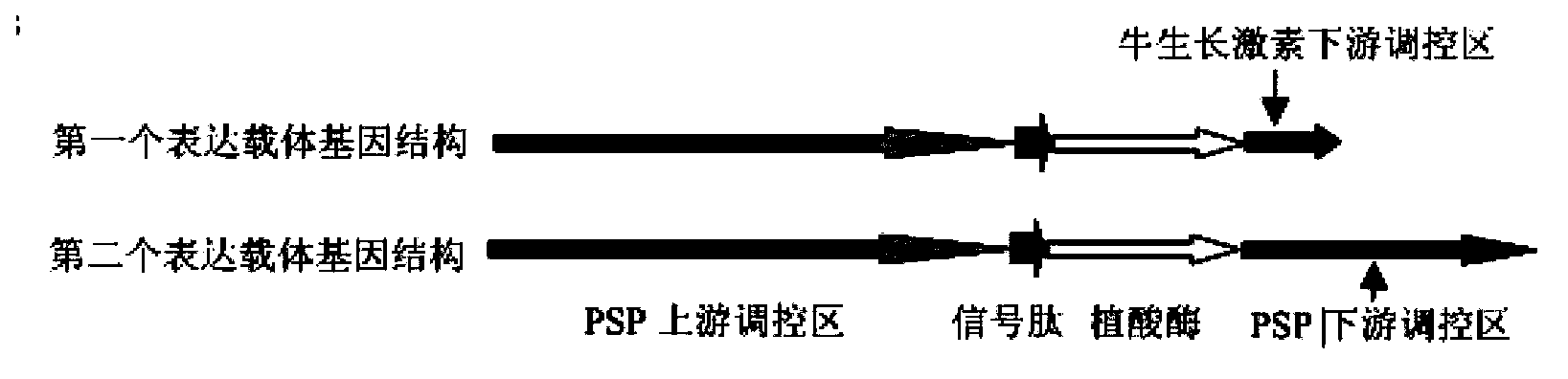
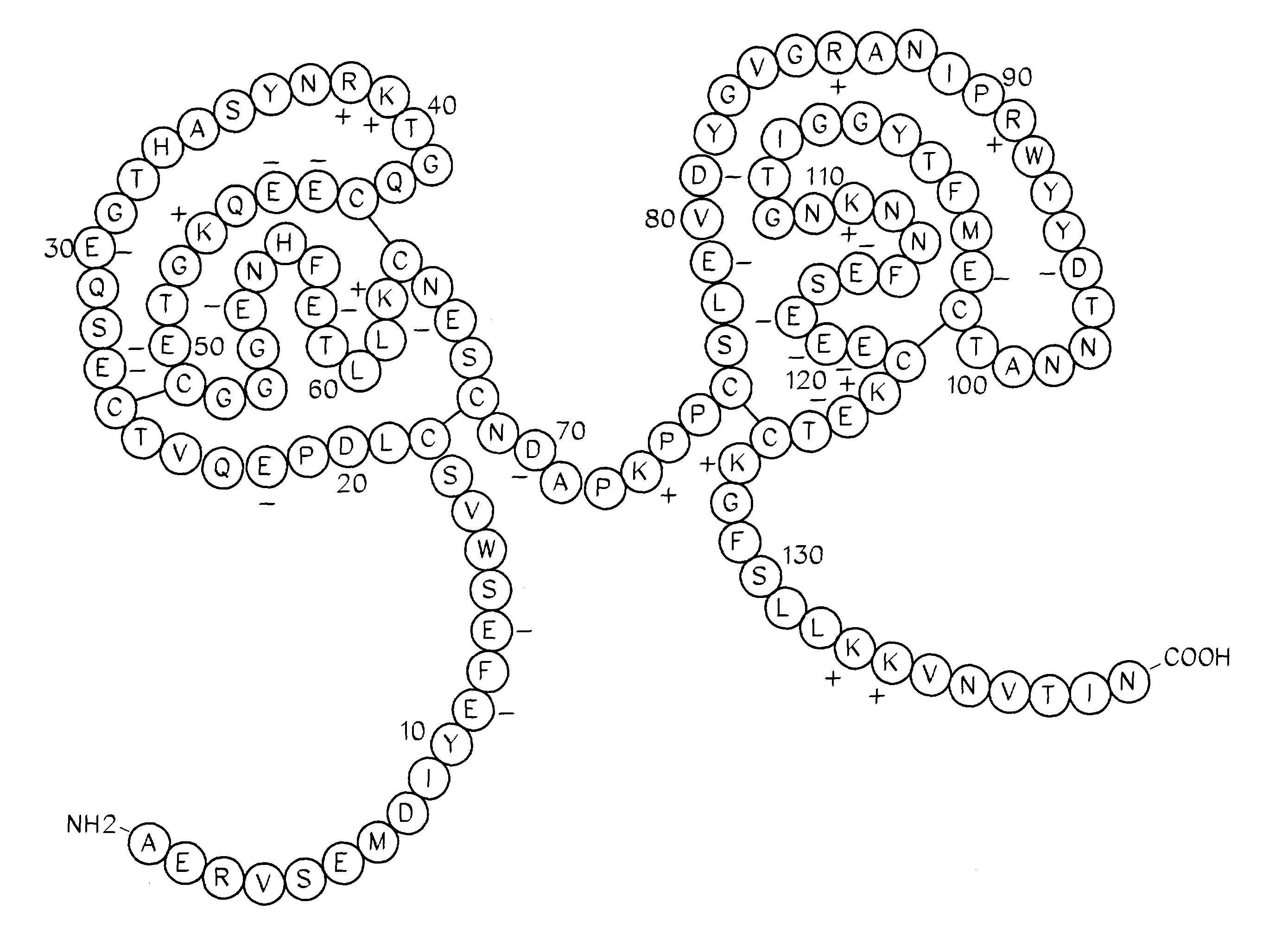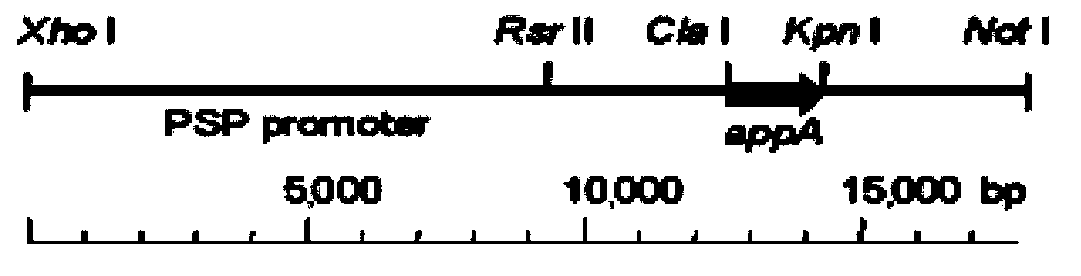Patents
Literature
177 results about "Salivary Gland Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Salivary glands can be classified as serous, mucous or seromucous (mixed). In serous secretions, the main type of protein secreted is ptyalin (alpha-amylase), an enzyme that breaks down starch into maltose and glucose, whereas in mucous secretions the main protein secreted is mucin, which acts as a lubricant.
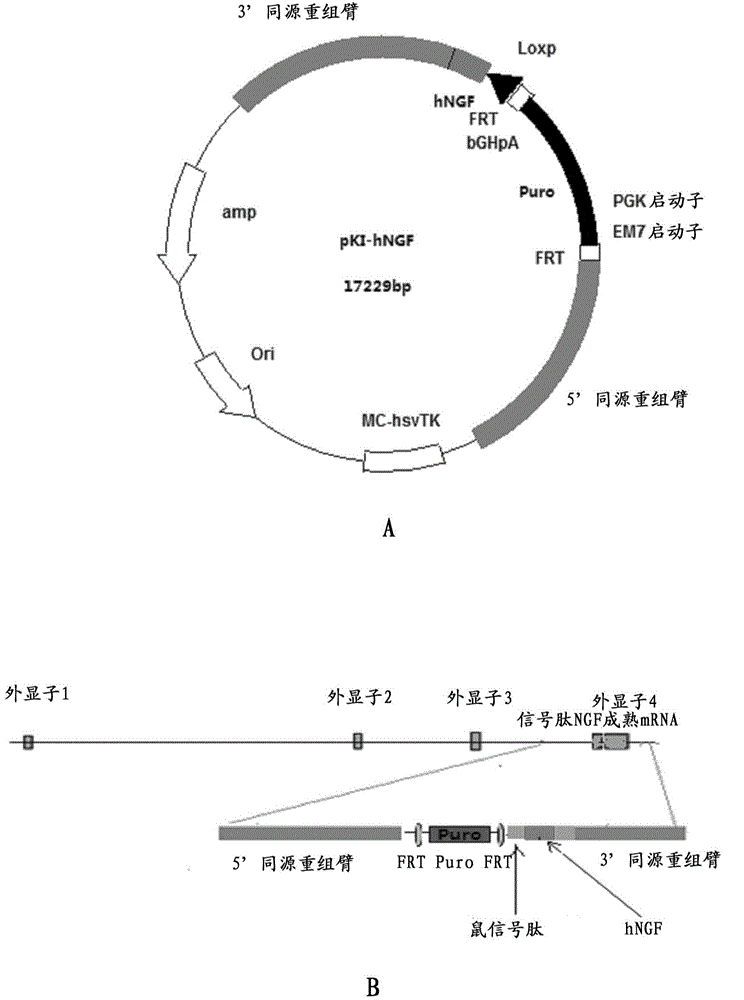
Preparation method for transgenic mice capable of producing human nerve growth factor
ActiveCN104561095AEasy to operateIncrease success rateAnimals/human peptidesVector-based foreign material introductionPlasmid dnaGenetically modified mouse
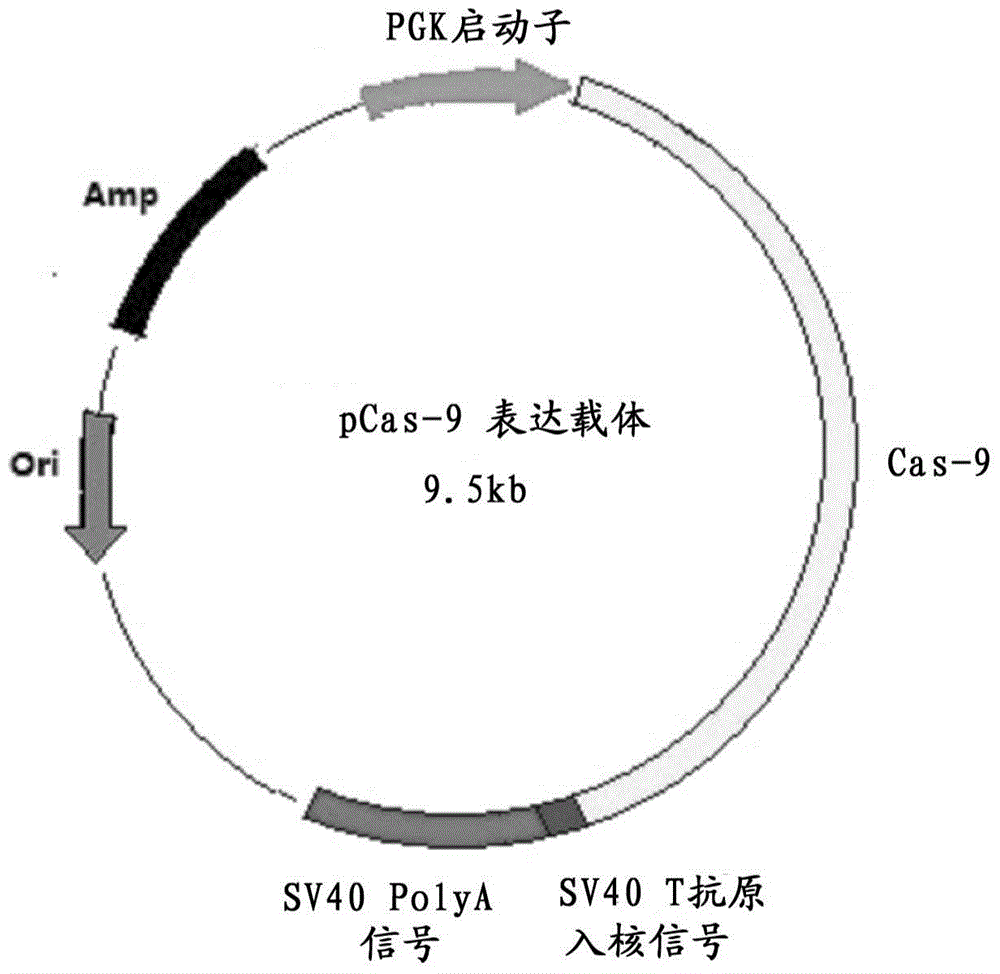
The invention discloses a method for producing transgenic mice through a homologous recombination technology. The method comprises the following steps: replacing mouse NGF genes on mouse chromosomes by human NGF genes through a Cas-9 / CRISPR gene knock-in technology, and obtaining the genes with homozygous human NGF genes through breeding and knocking the genes in mice, thus obtaining filial generation mice with salivary glands capable of secreting human NGF. Compared with the conventional targeting technology utilizing positive and negative double screening homologous recombination genes, the method disclosed by the invention is simple to operate, capable of being realized only by transfecting mouse embryonic stem cells by three plasmid DNAs or carrying out pronucleus injection on the zygotes of mice, and high in success rate which is up to 2-5% and remarkably higher than the mouse embryonic stem cell positive rate of 0.1% of the common gene targeting technology.
Owner:深圳市国创纳米抗体技术有限公司
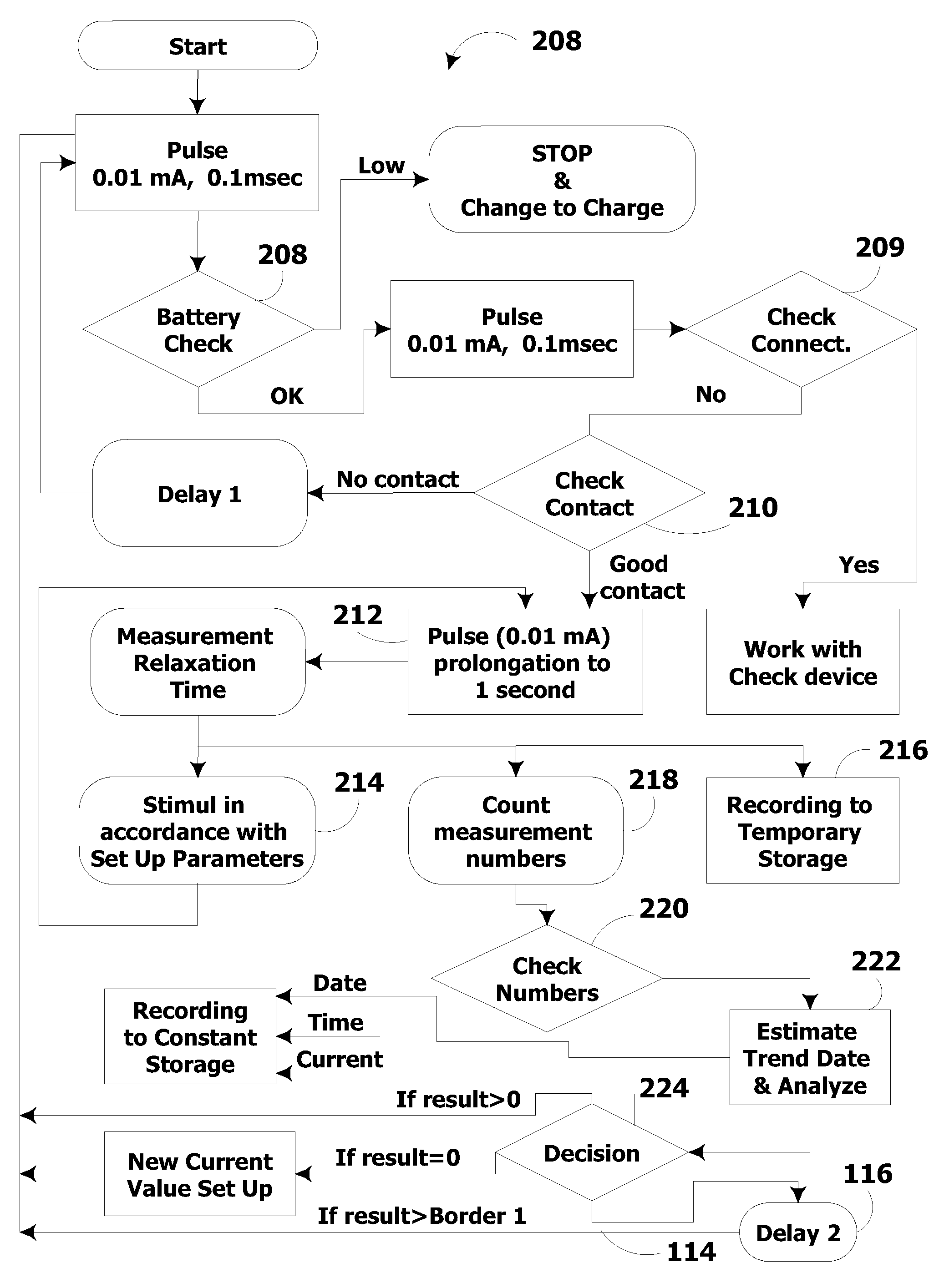
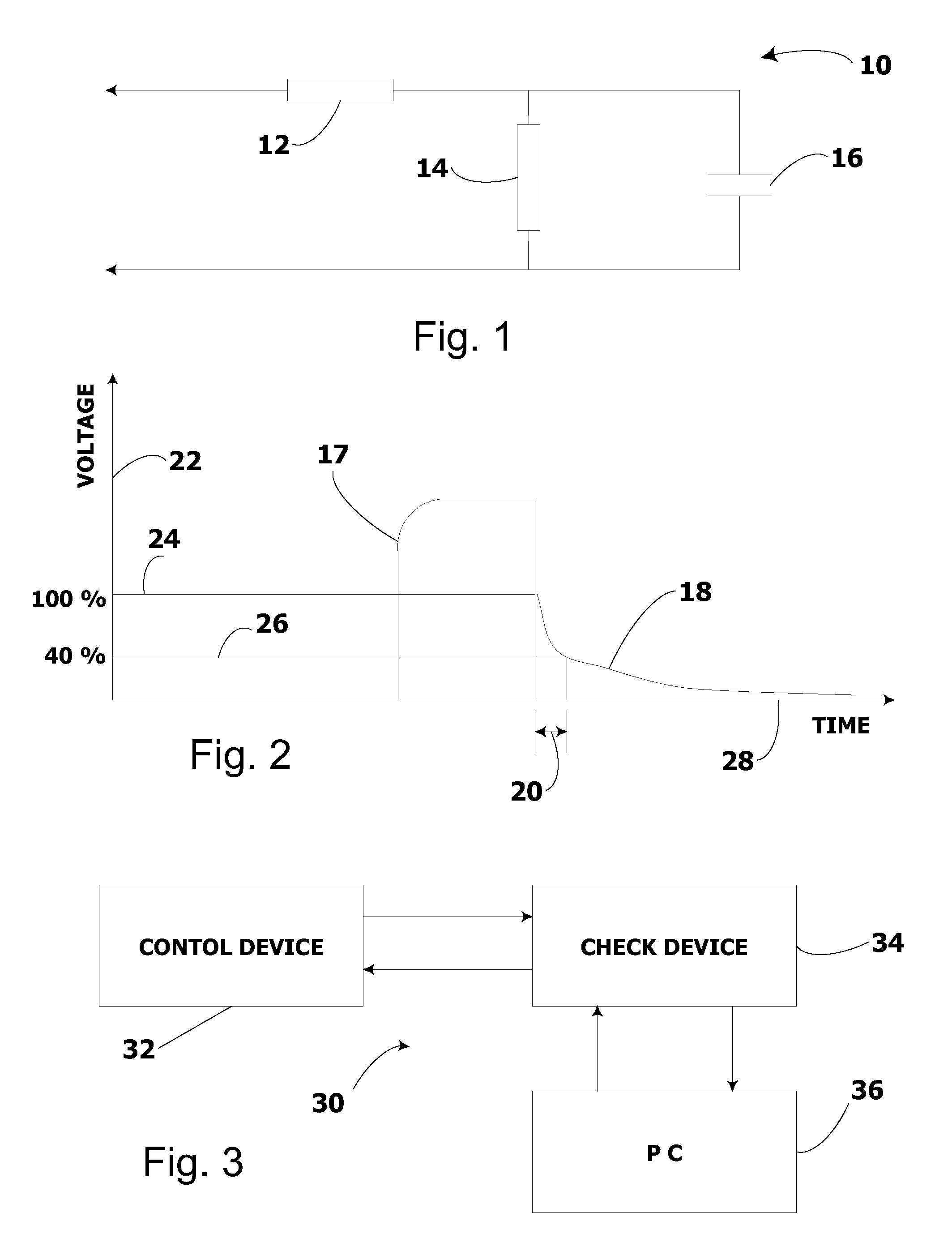
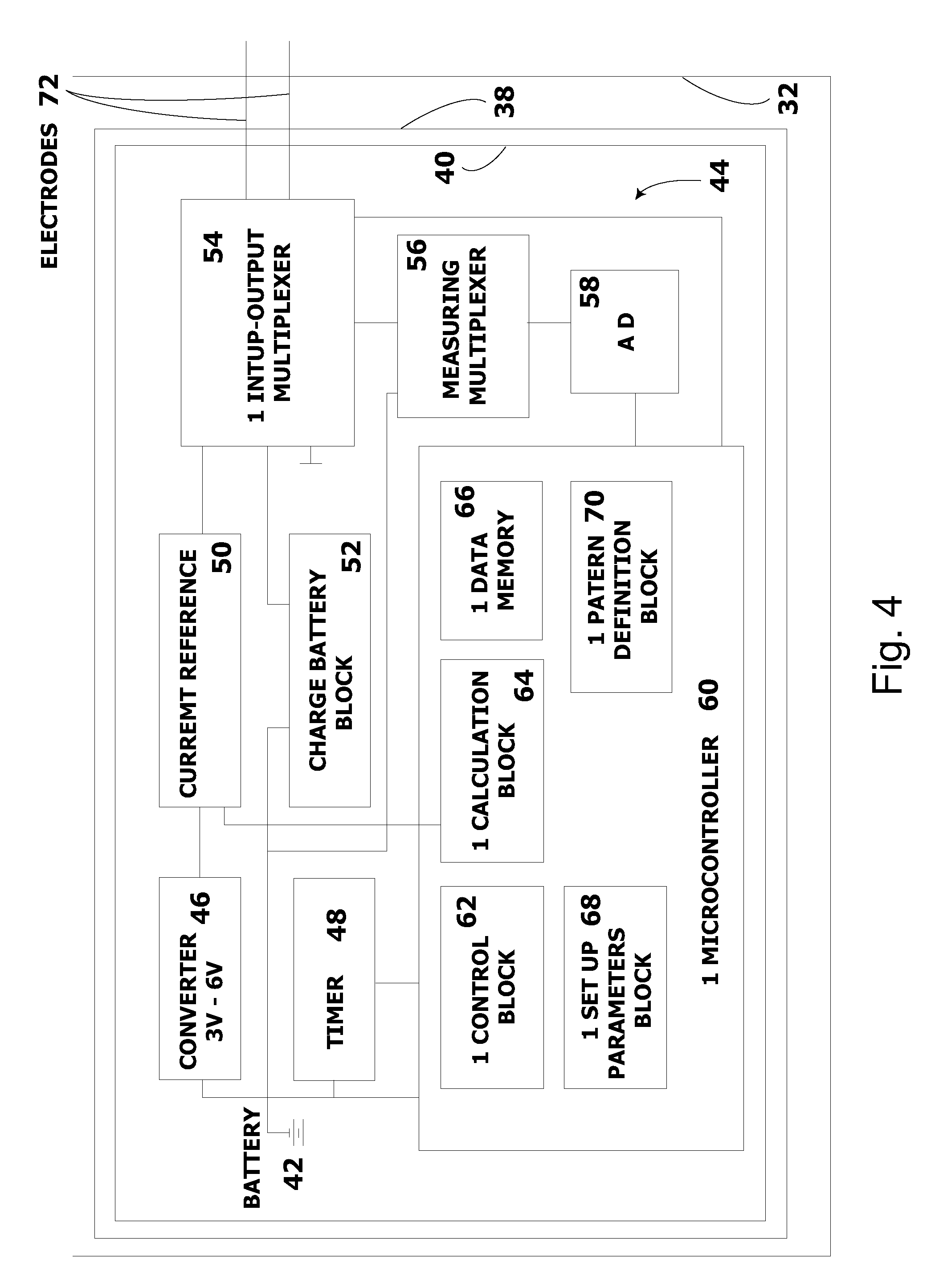
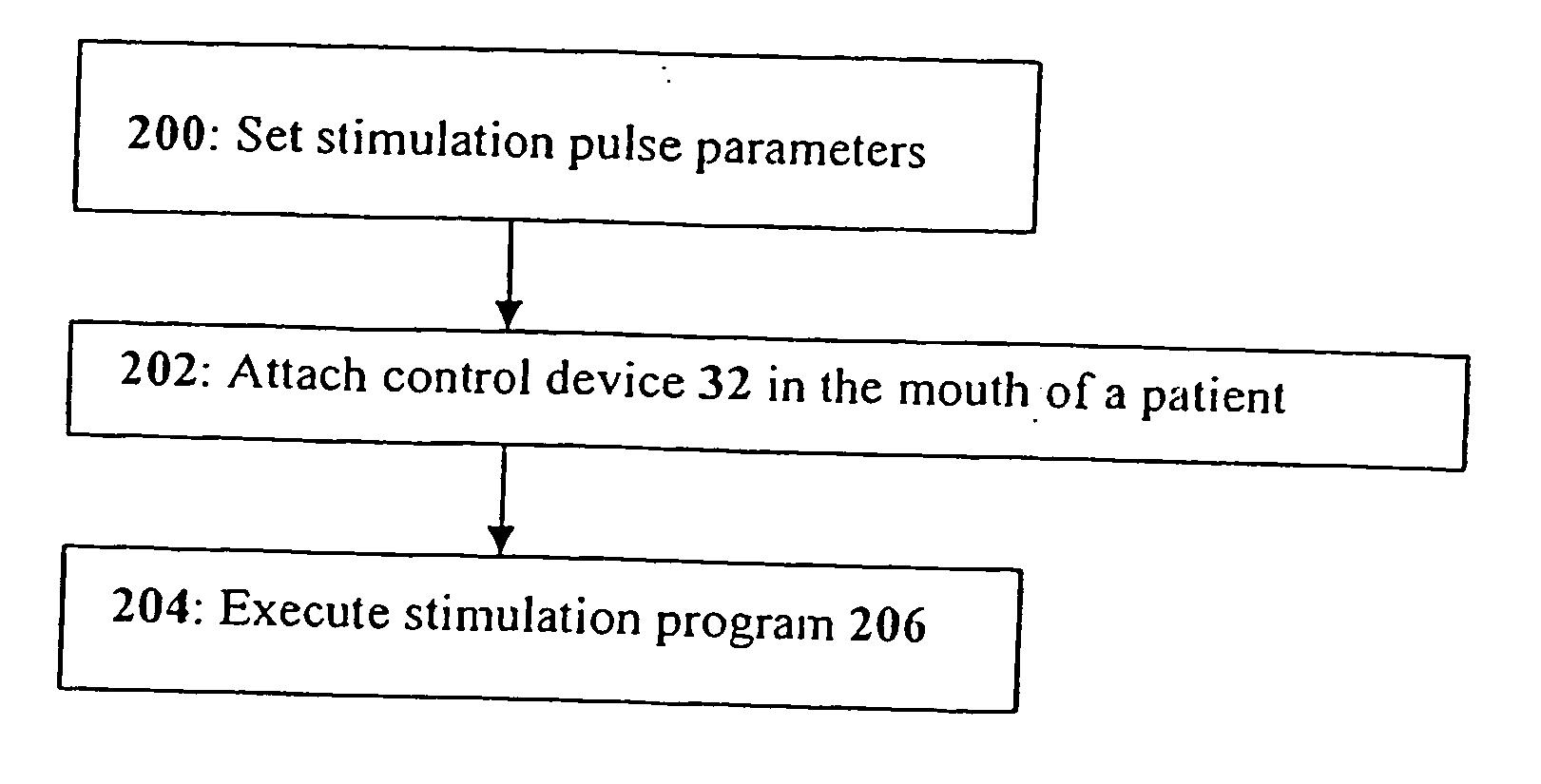
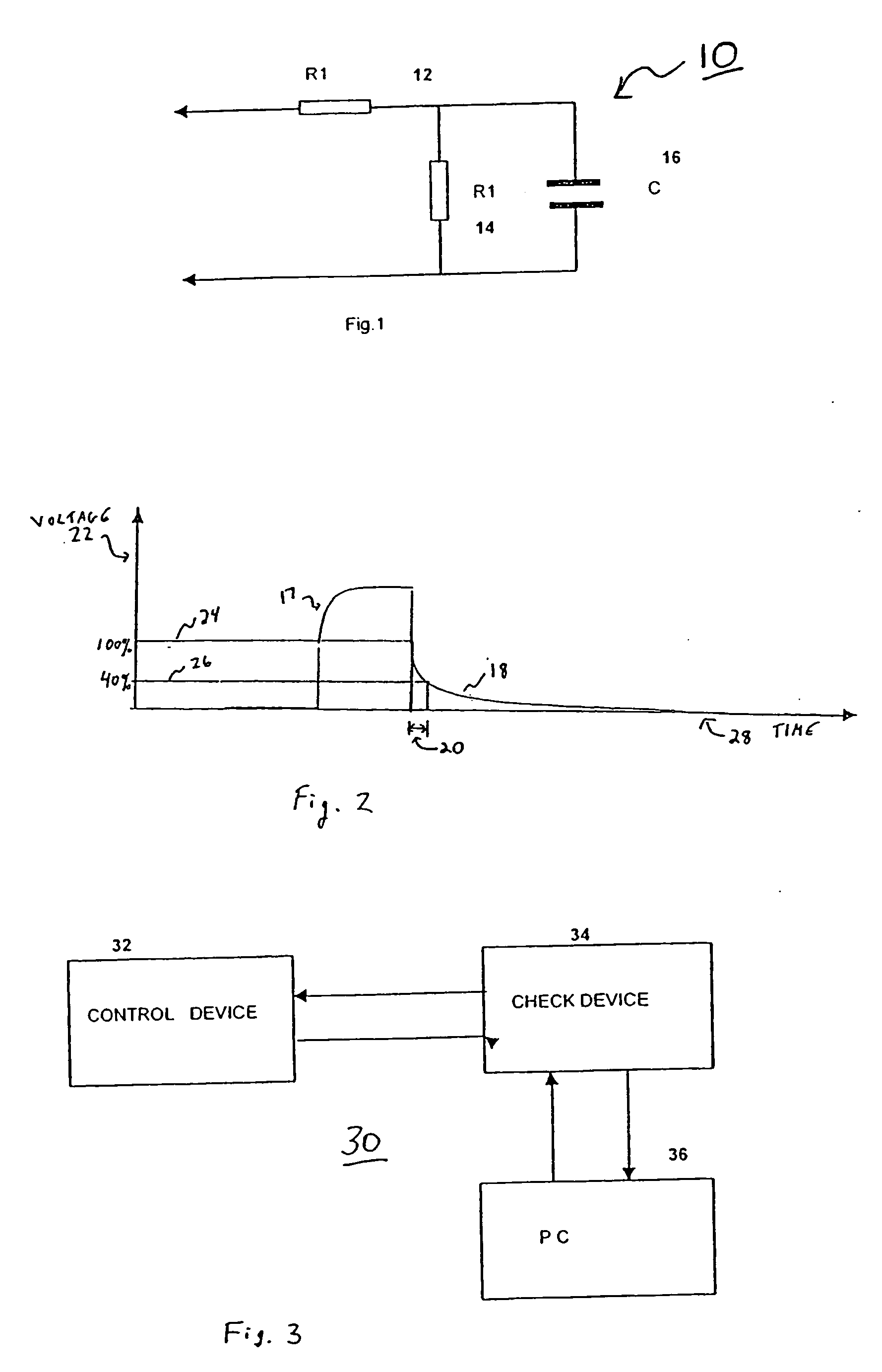
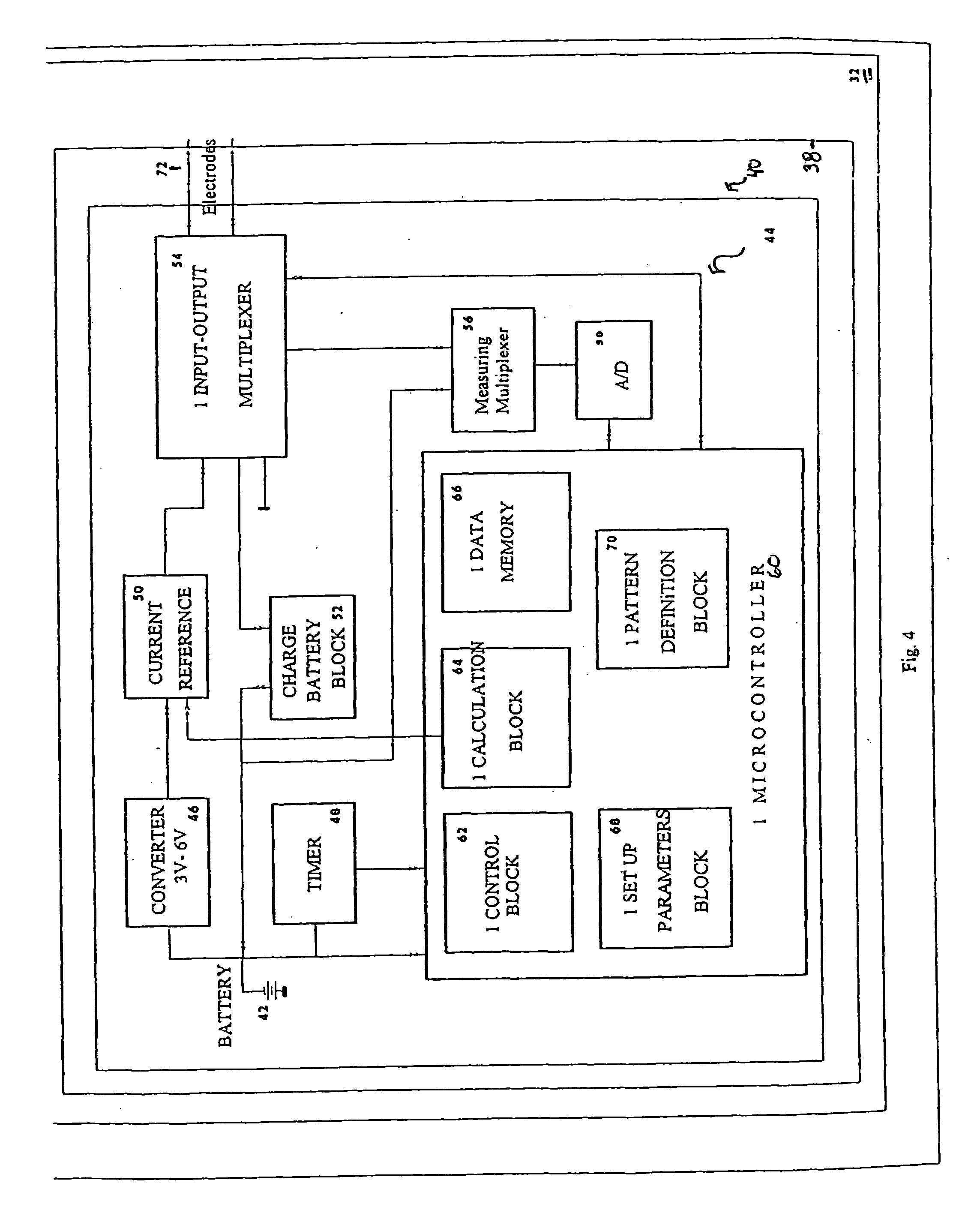
System and method for electrical stimulation of salivation
A system, a device and a method for electrically detecting a lack of saliva in an oral cavity of an individual and for electrically stimulating the oral cavity so as to induce production of saliva from at least one salivary gland are disclosed. The system includes a control device for detecting a measure of salivation in the individual and for delivering electrical impulses to the oral cavity of the individual, a check device for checking a state of the control device and for modifying at least one parameter of the control device, and, a computer device for exchanging information with the check device.
Owner:SALIWIZER ITD
Therapy for the treatment of disease
ActiveUS20070053995A1Relieve constipationBiocideNervous disorderAnticholinergic agentsCompound (substance)
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Fusion protein for treating diabetes, and its preparing method and use
InactiveCN1935846AImprove efficacyEasy to separate and purifyOrganic active ingredientsMetabolism disorderHalf-lifeFactor ii
The invention relates to fusion protein used to prevent and cure I type and II type diabetes and its preparation method and application. It supplies the fusion protein which is formed by glucagon analogy peptide GLP-1, GLP-1 mutant and IgG-Fc, its analogy factor lizard salivary gland polypeptide extendin-4 and IgG-Fc, and the application of the above fusion protein and its DNA used in diabetes prevention and therapy. The fusion protein can increase not only GLP-1 effect, but also the affinity and immunological tolerance of ligand, which is excreted with the form of homogeneity dimmer to improve polypeptide drug effect, overcomes the defect of the GLP-1 for short half-life, and simplifies purification process.
Owner:王庆华 +1
Use of gaseous nitric oxide as an anti-cancer agent
InactiveUS20070275100A1Effectively deliver gaseous NOEfficient procedureBiocideBronchoscopesCell phenotypeAnticarcinogen
The invention relates to a method for treating, controlling, or preventing cancerous cell phenotypes and growths in an animal involving the administration of gaseous nitric oxide to one or administration sites in a body. The invention generally is capable of treating cancers found in or on the adrenal gland, bladder, bones, brain, breast, cervix, colon, colorectum, esophagus, gastrointestinal tract, heart, kidney, liver, large intestine, lungs, mouth, ovaries, pancreas, parathyroid, pituitary gland, prostate, salivary gland, skin, small intestine, spleen, stomach, thymus, thyroid, testicles, urinary tract, uterus, vagina, and so forth.
Owner:PULMONOX TECH
Therapy for the treatment of disease
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Suction Lithotripsy Apparatus, Method and Kit
Owner:COOK MEDICAL TECH LLC
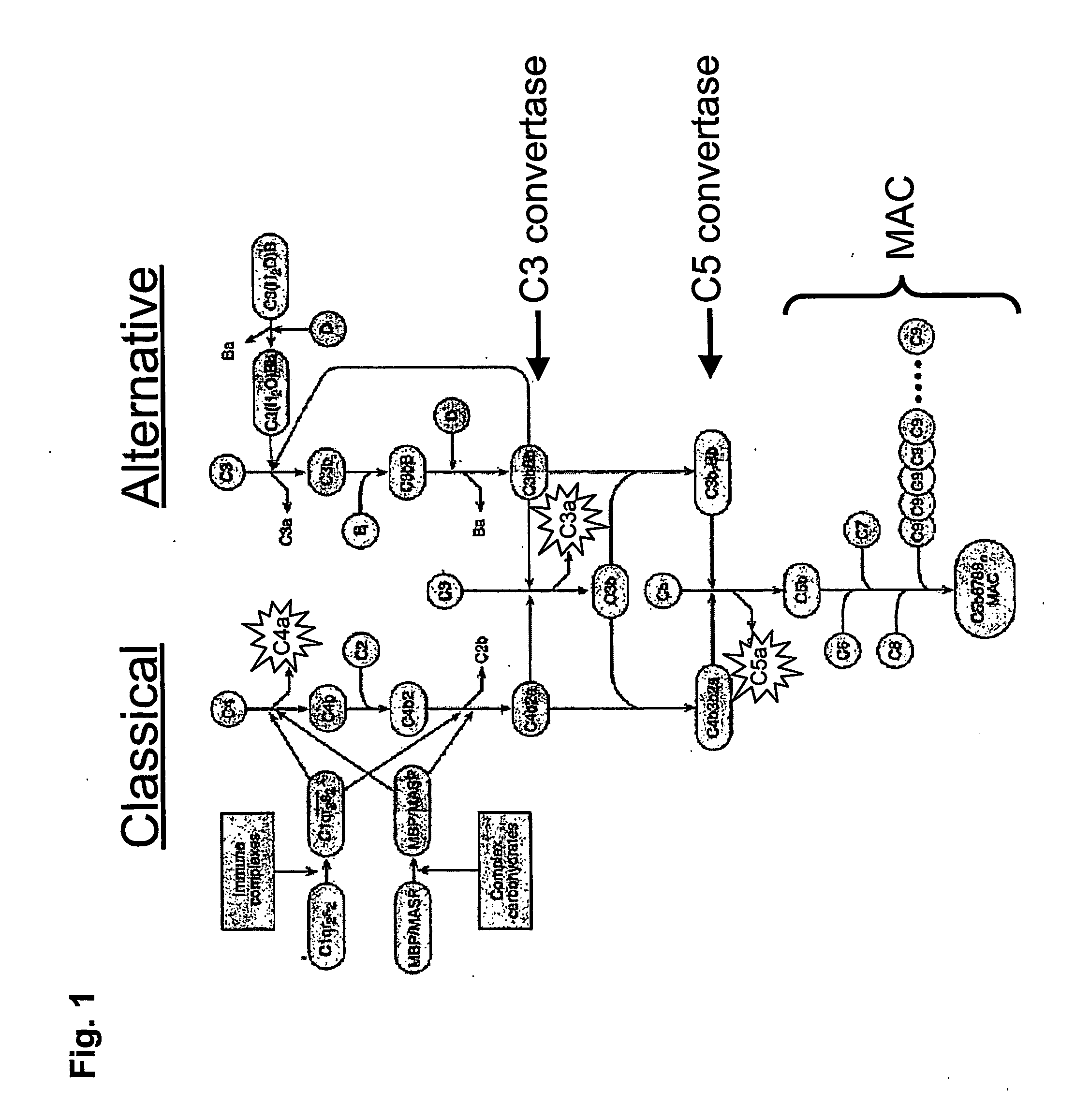
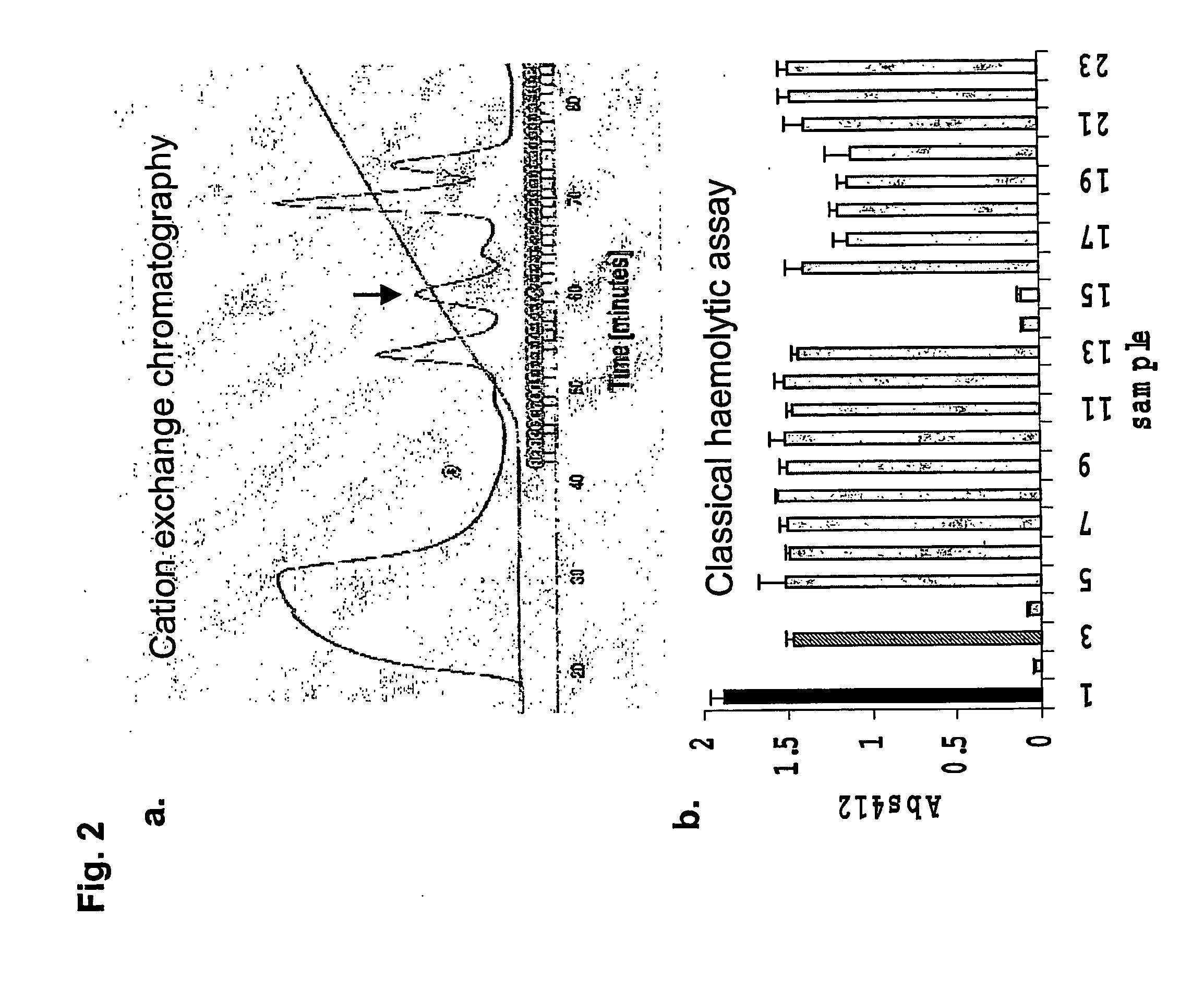
Complement inhibitors
ActiveUS20070141573A1Increase deposition rateConvenient treatmentAntibacterial agentsPeptide/protein ingredientsDiseaseMedicine
The invention relates to complement inhibitors that inhibit both the classical and alternative complement pathways. In particular, the invention relates to complement inhibitors derived from the salivary glands of haematophagous arthropods that inhibit both the classical and alternative complement pathways. The invention also relates to the use of such complement inhibitors in the treatment and prevention of diseases.
Owner:VOLUTION IMMUNO PHARMA
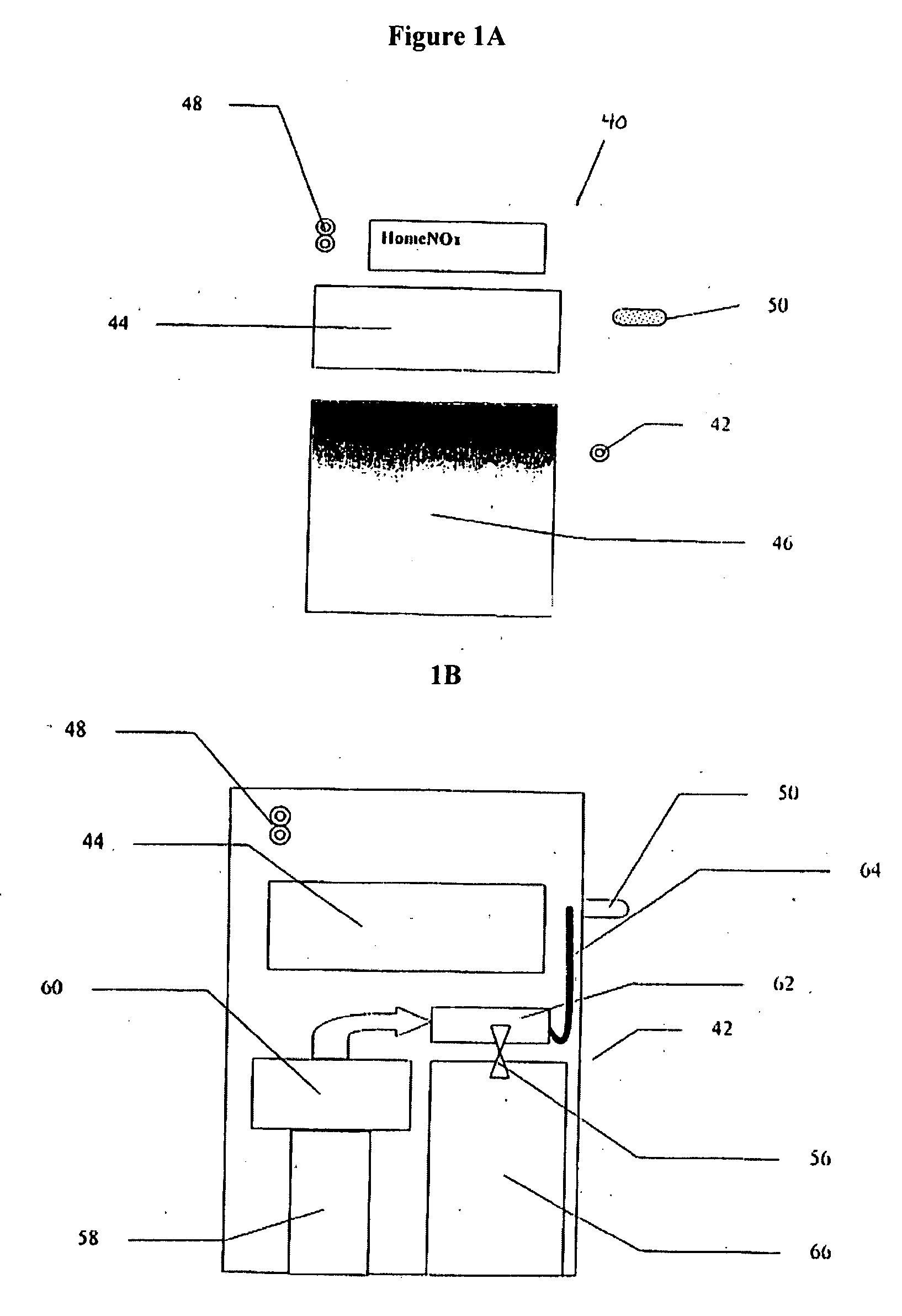
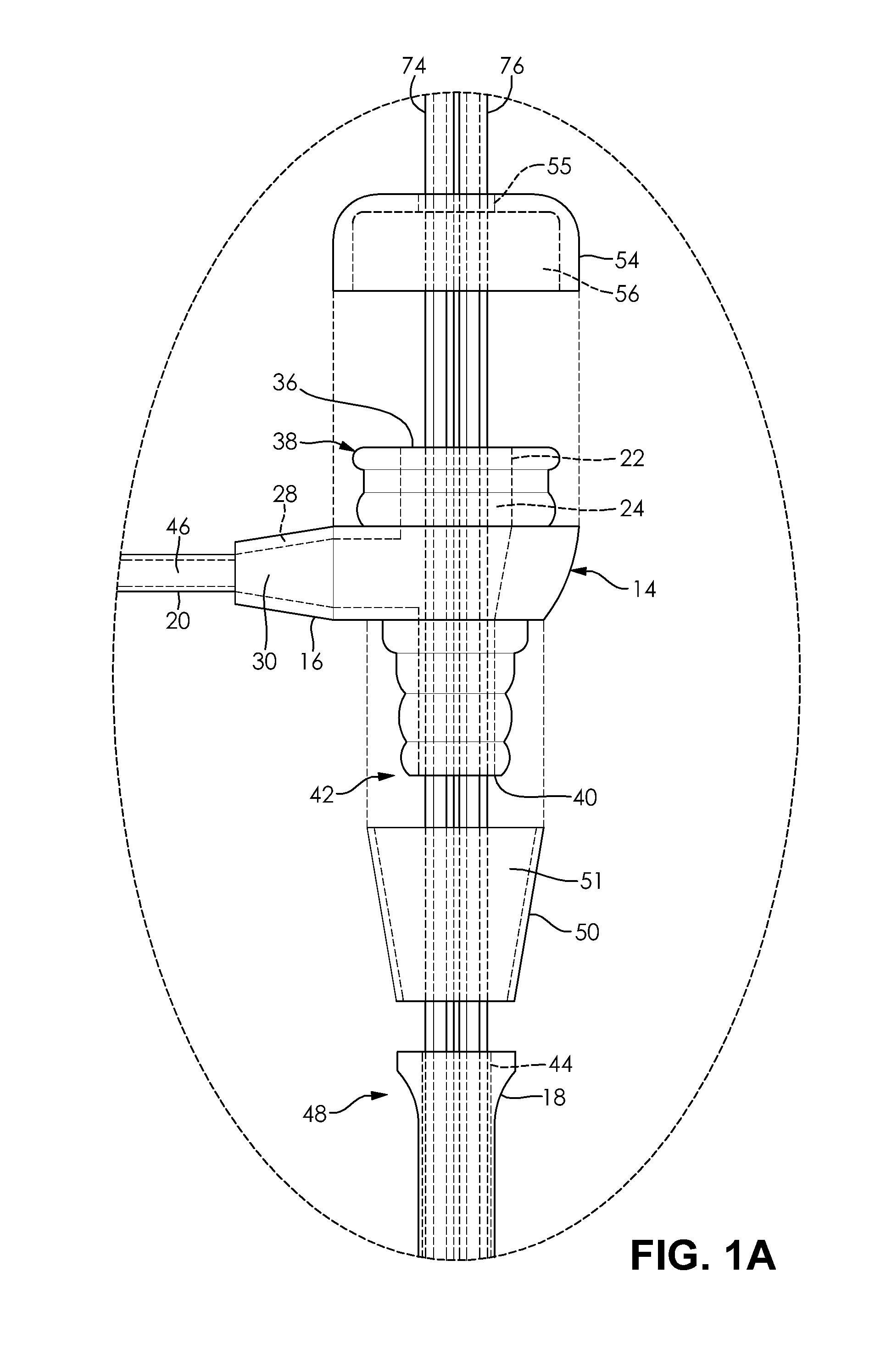
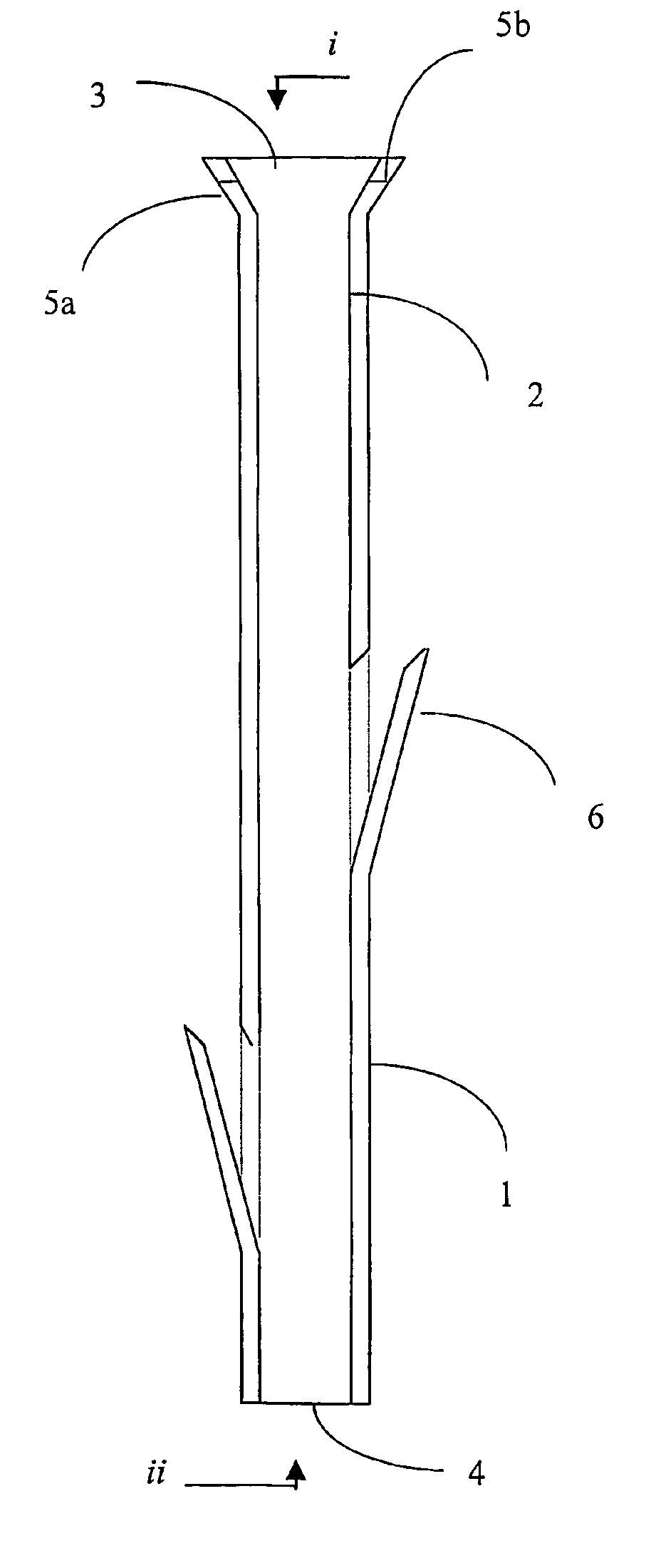
Polymeric stent useful for the treatment of the salivary gland ducts and method for using the same
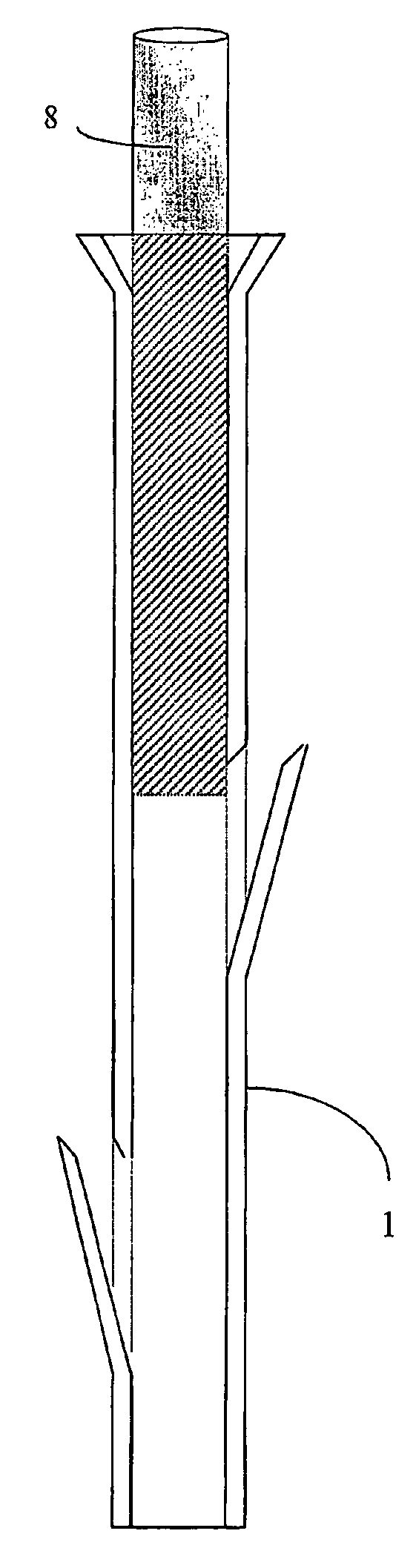
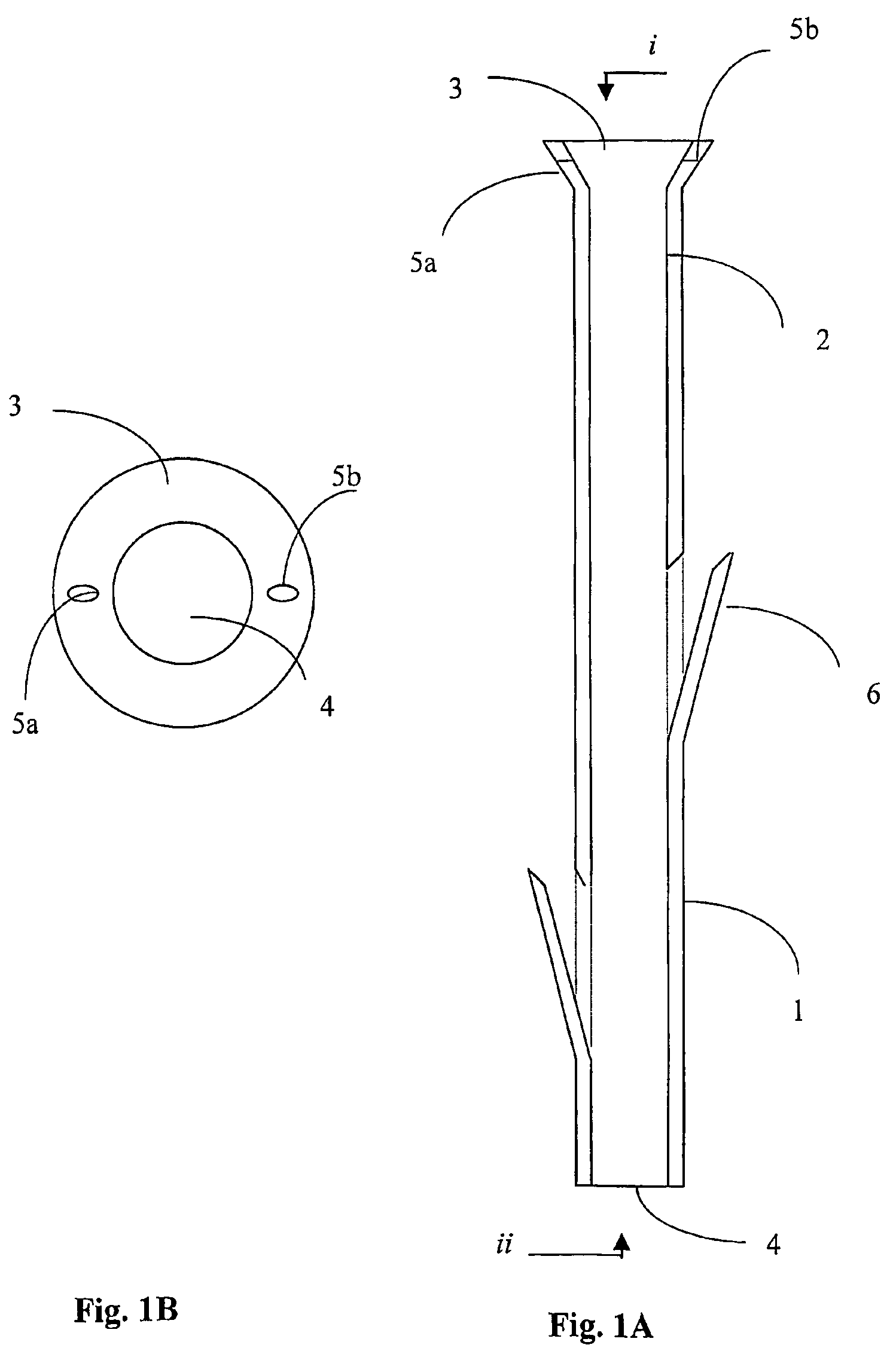
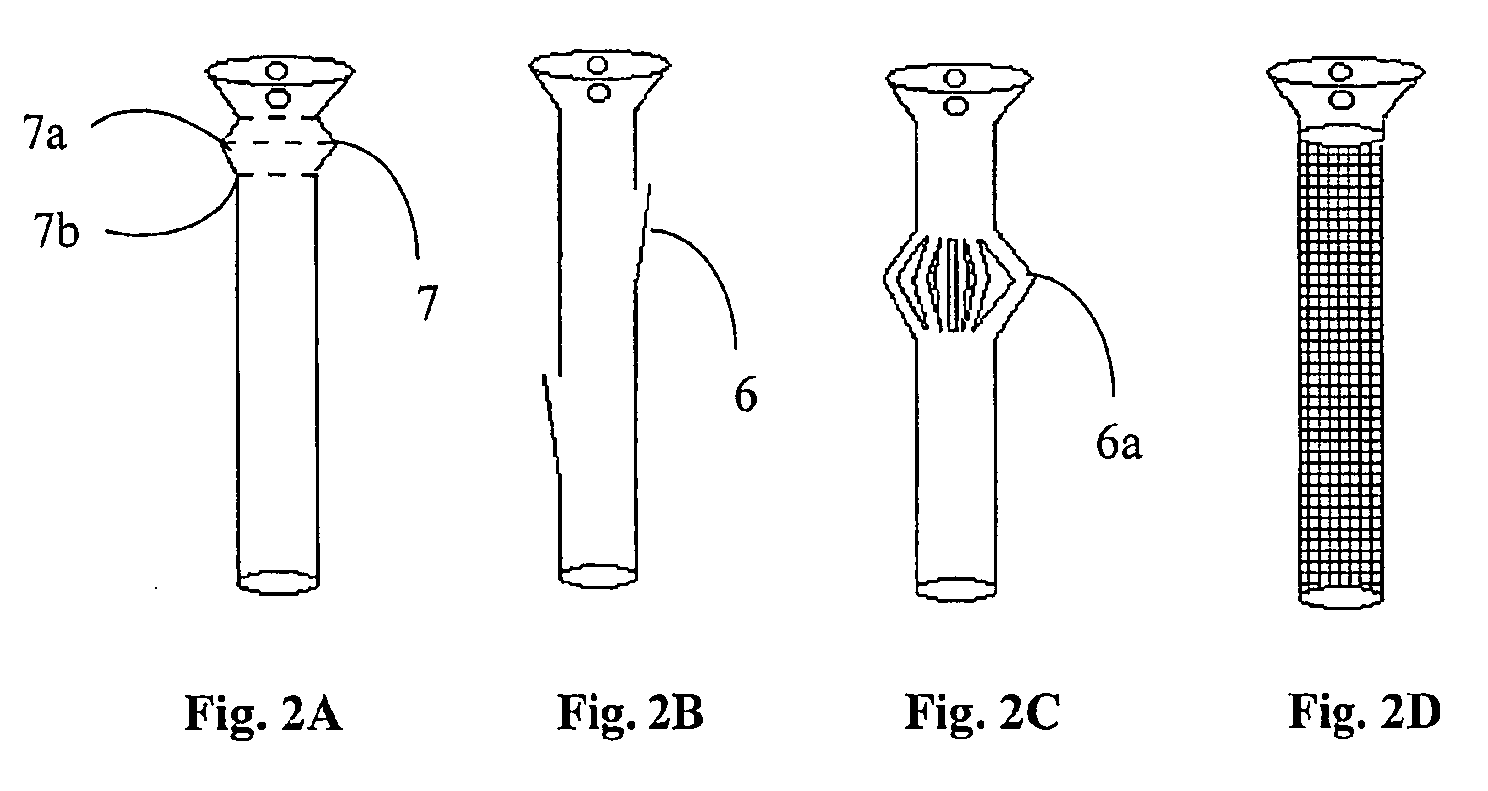
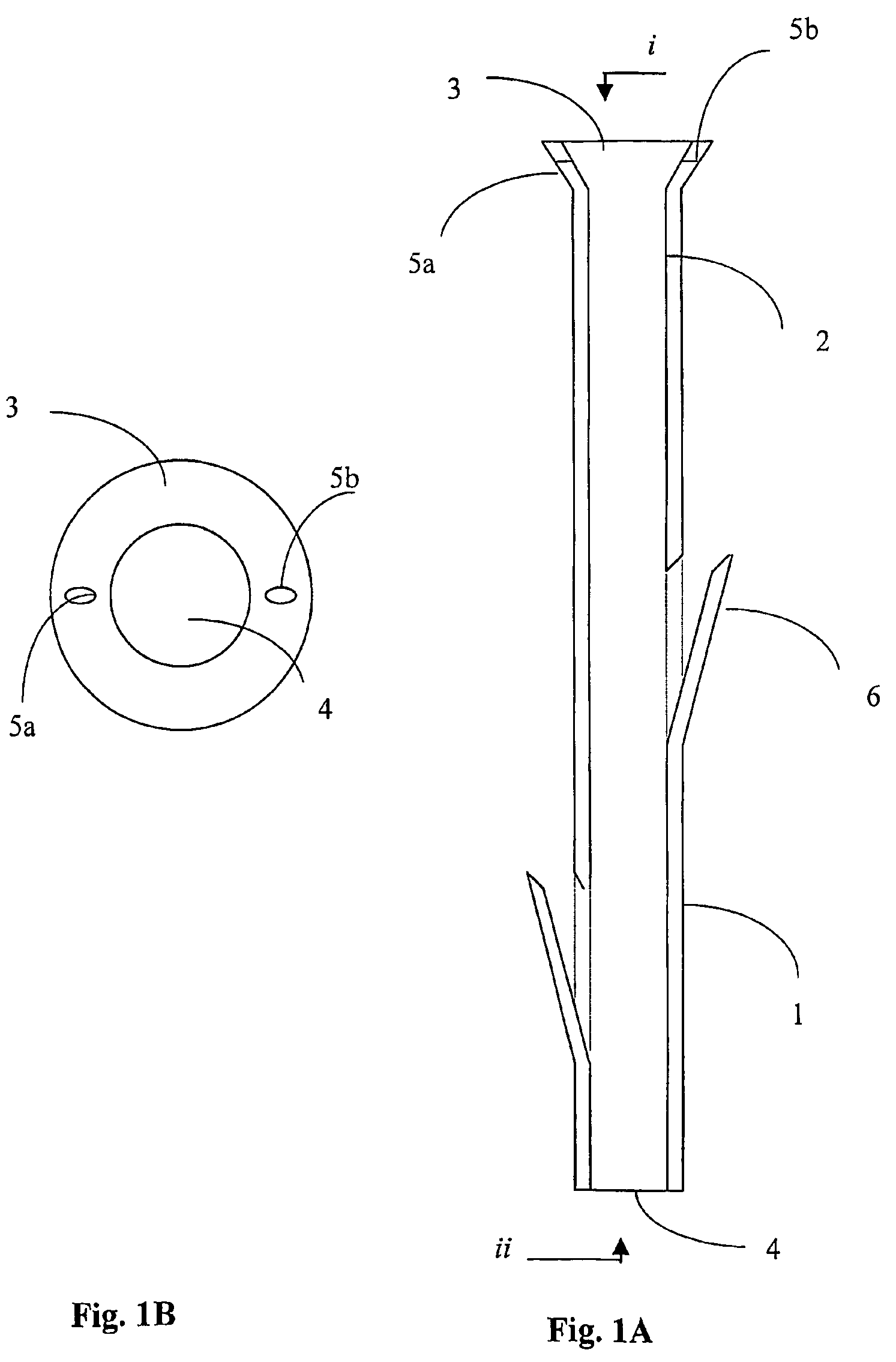
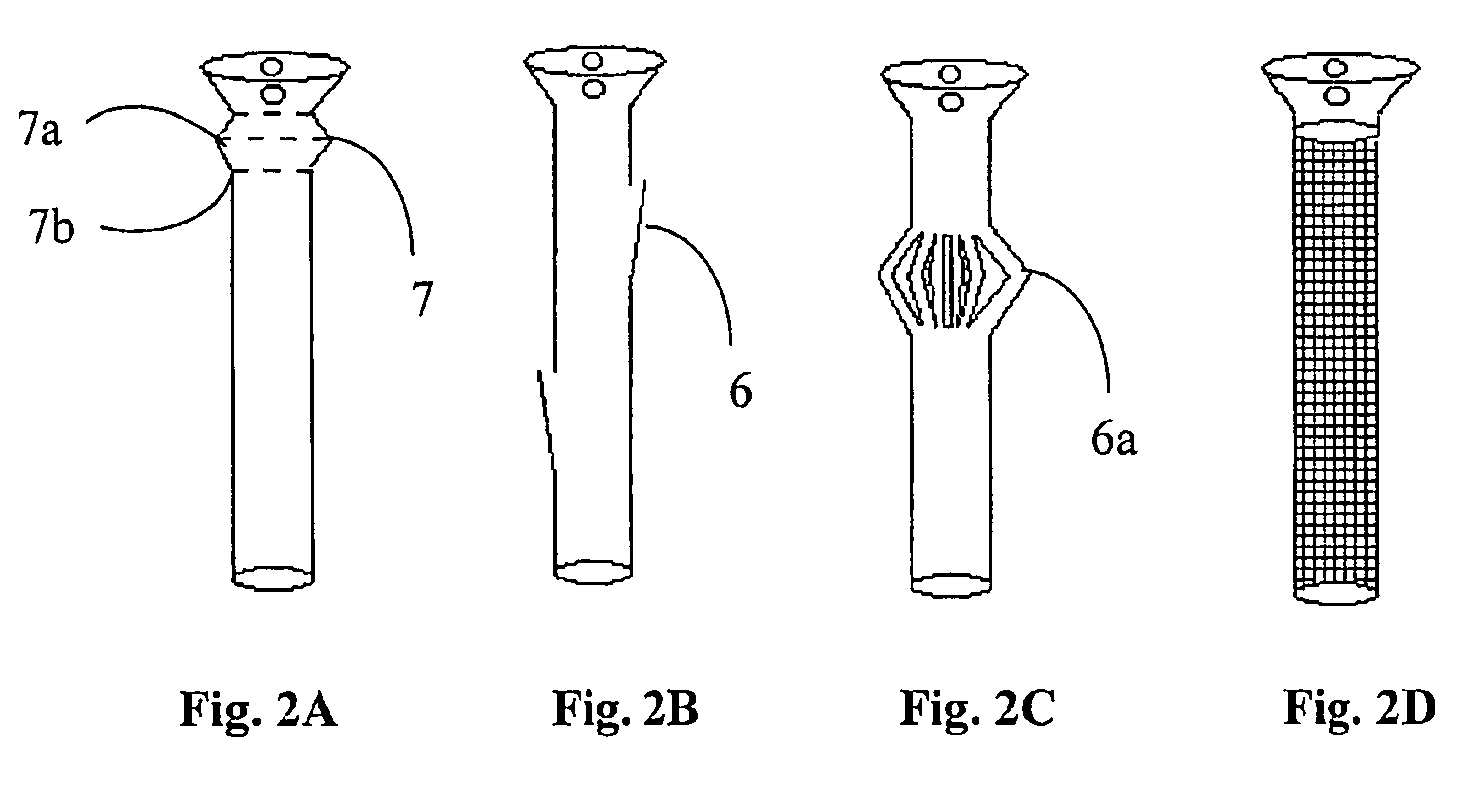
A polymeric stent, especially useful in surgical endoscopy and for the treatment of salivary gland ducts comprising; an elongated tube (1), wherein the proximal end (3) of said tube is having a funnel-like shape; and wherein said funnel further comprise at least one gorge (5a), which enables the suturing of said stent to said duct. The invention also relates to a method for implanting the polymeric stent into the lumen of a salivary gland duct.
Owner:NAHLEILI ODED
Serum-free culture fluid for culture of salivary gland epidermal cell and salivary gland stem cell of mammals
ActiveCN102399742AProliferation effect is goodGood biological propertiesArtificial cell constructsArtificially induced pluripotent cellsCulture fluidAntioxidant
The invention provides serum-free culture fluid for culture of salivary gland epidermal cells and salivary gland stem cells of mammals, which is prepared by using MCDB153HAA as basic culture medium and adding amino acid, vitamins, salts, lipid, trace elements, buffer fluid, hormone-like compounds, transgenic metalloprotein, antioxidants, seralbumin, glucide, purine, pyrimidine base substances and pH value indicators. The serum-free culture fluid has the advantages that salivary gland epidermal cells of the mammals can vigorously grow, good cell activity and physiological properties are maintained, in addition, the serum-free culture fluid is also suitable for the salivary gland epidermal cell culture, is particularly suitable for the field of study relevant to biological tissue engineering and belongs to the commercial cell culture fluid.
Owner:INST OF DONGGUAN SUN YAT SEN UNIV
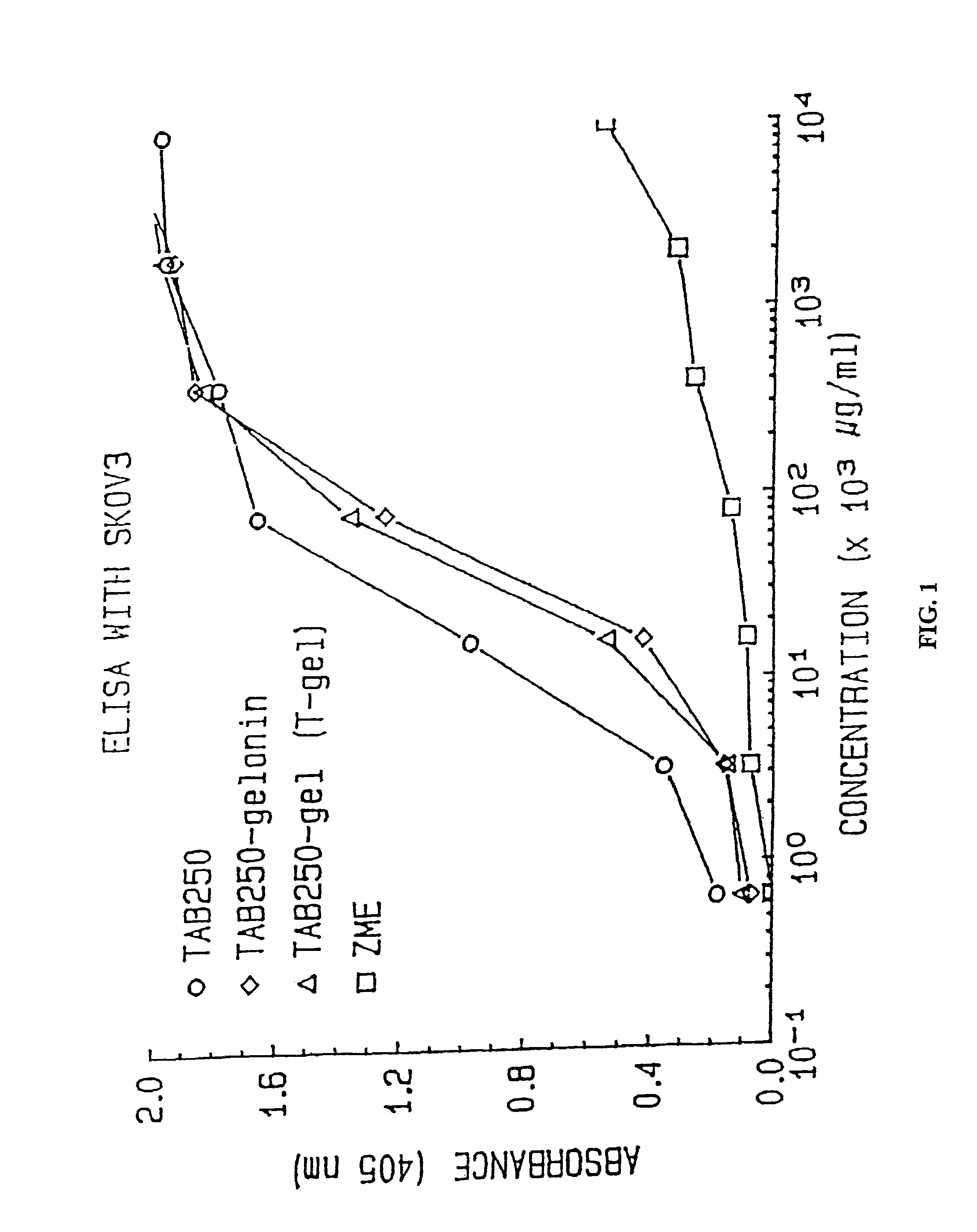
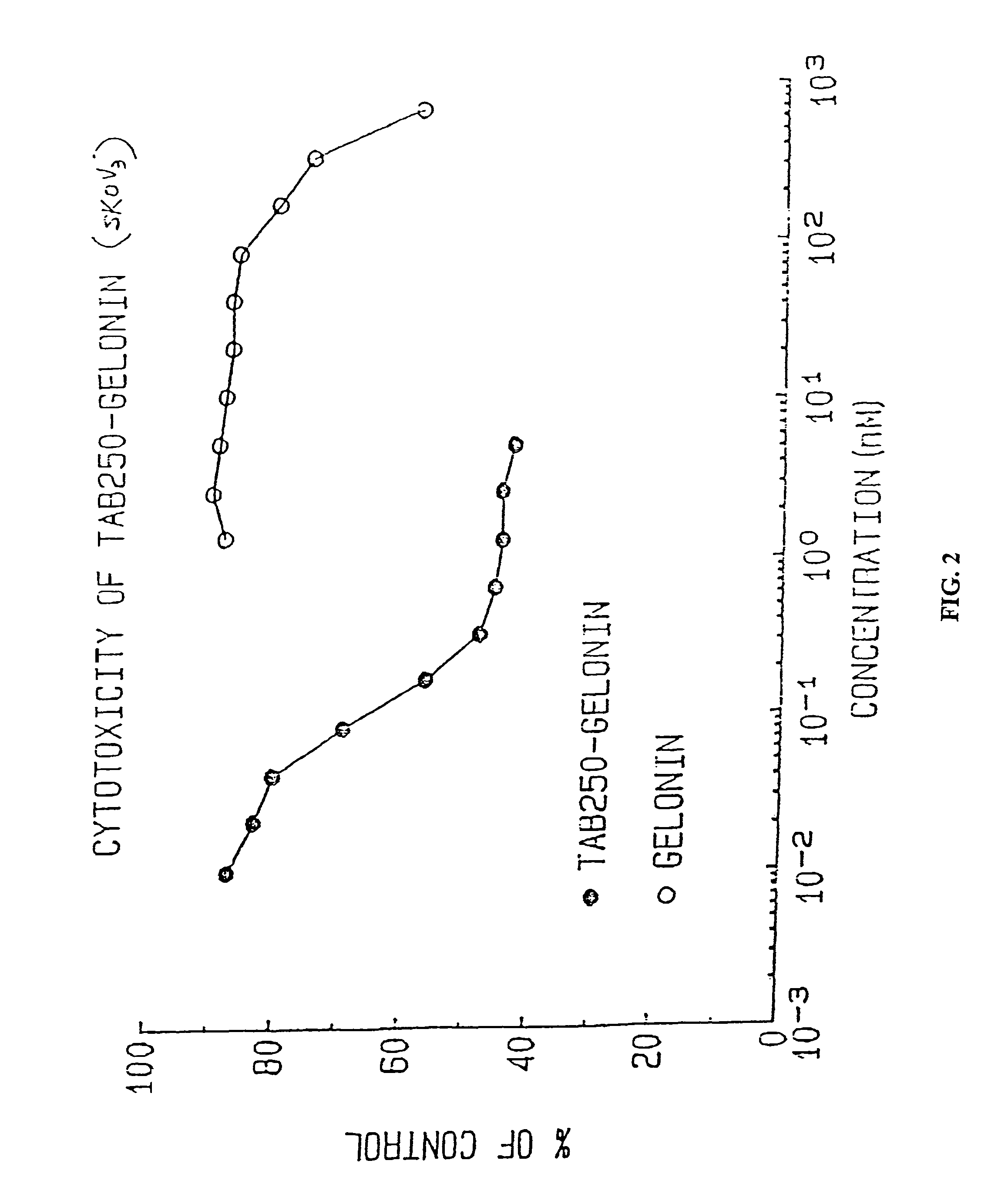
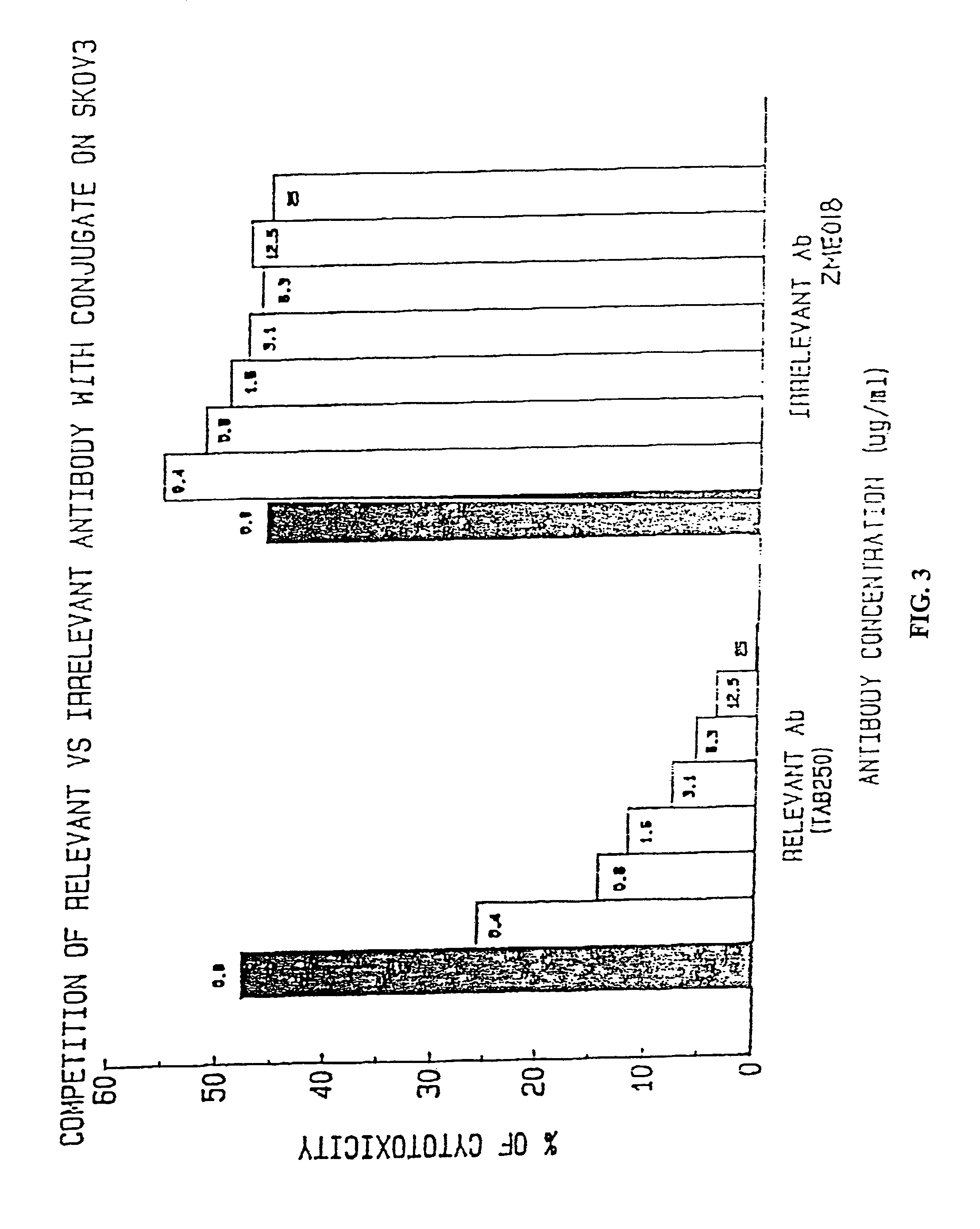
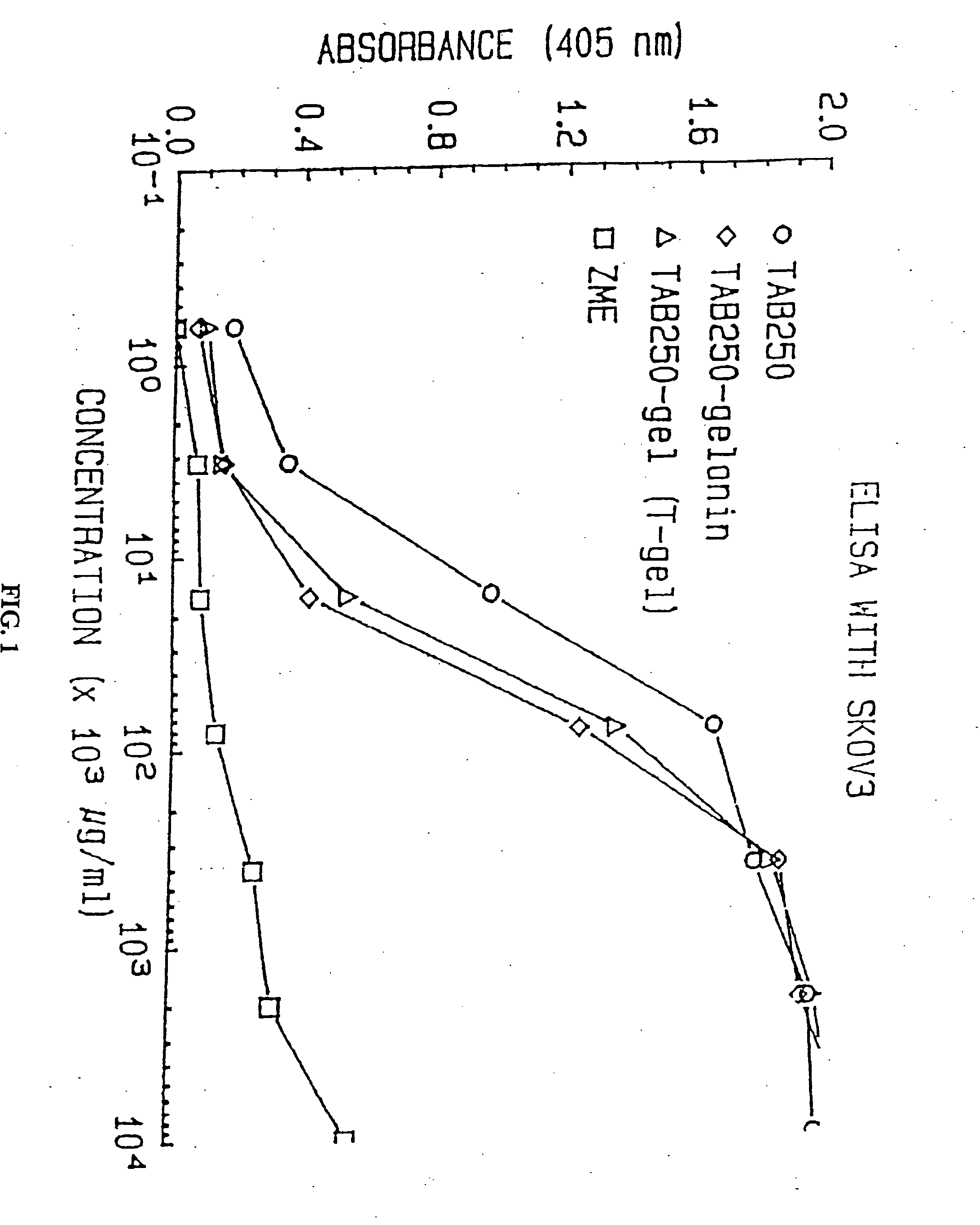
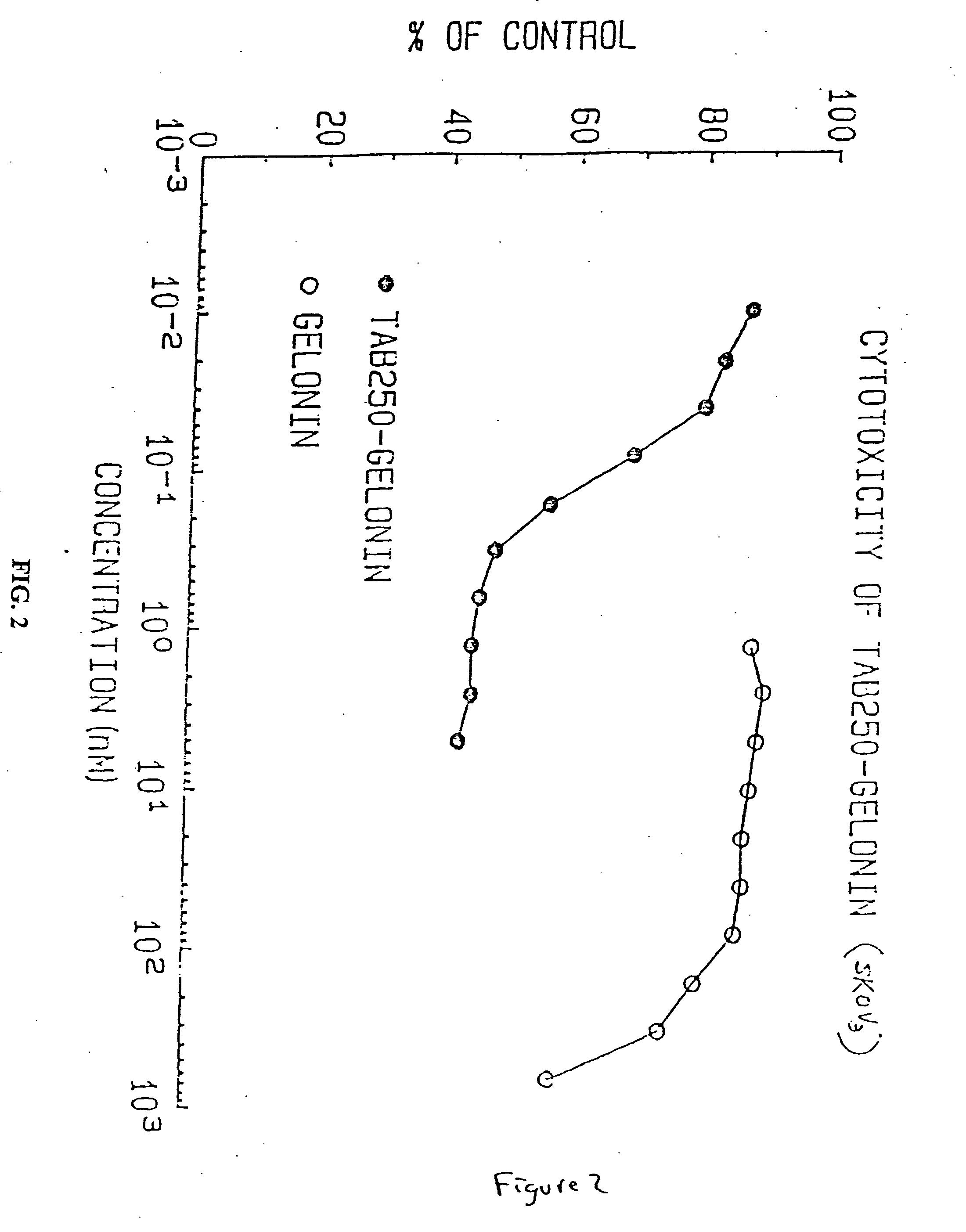
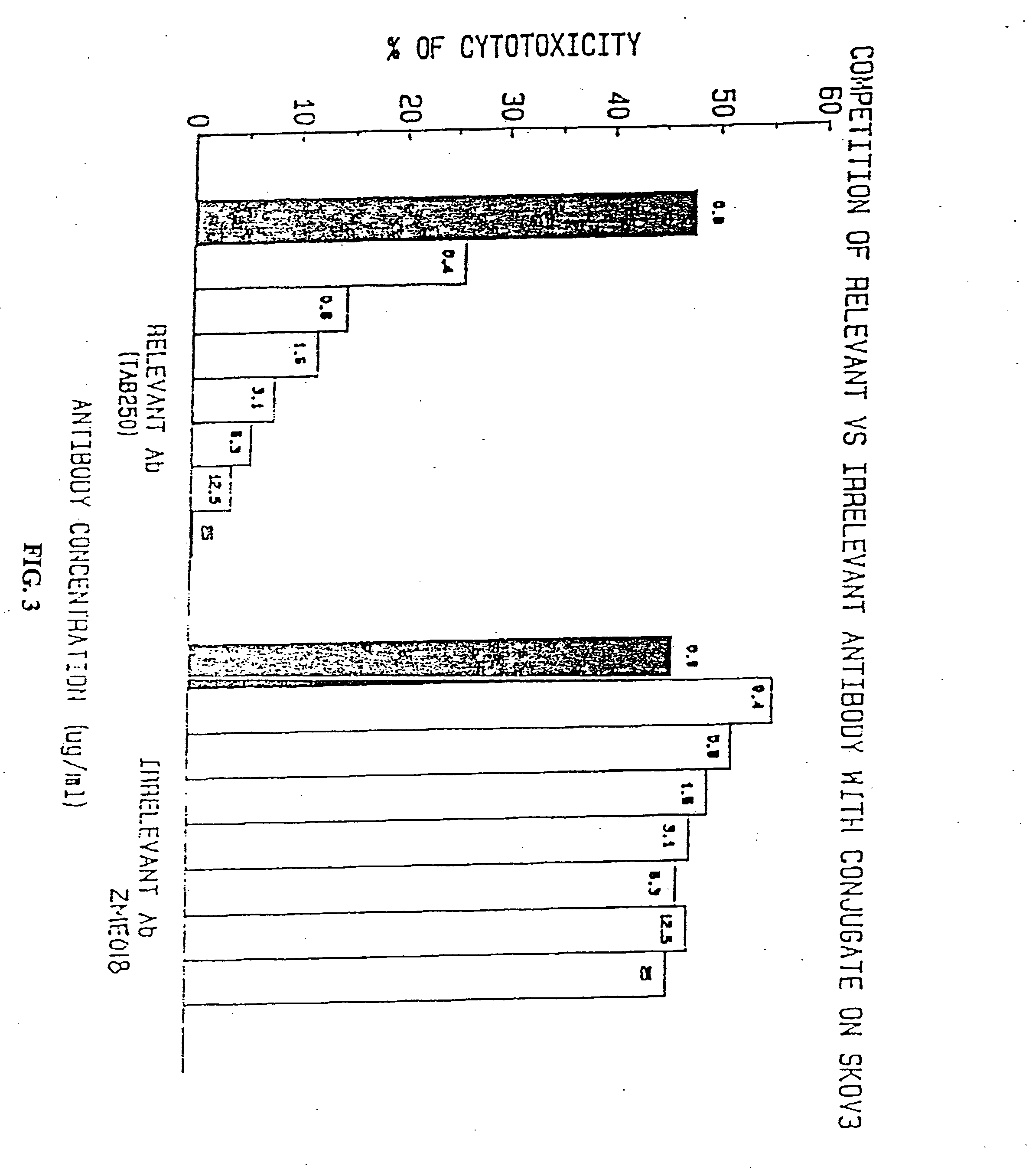
Immunotoxins directed against c-erbB-2(HER-2/neu) related surface antigens
Novel immunotoxins and methods of treating neoplastic diseases are provided. More specifically, immunotoxins comprised conjugation of a c-erbB-2 targeting moiety and a cell growth modulator are provided. These immunotoxins specifically and selectively kill tumor cells that over-express the c-erbB-2 protein. The novel immunotoxins would be useful in treating human mammary carcinomas, human ovarian carcinomas, lung carcinomas, gastric tumors, salivary gland adenocarcinomas, and colon adenocarcinomas.
Owner:RES DEVMENT FOUND
System and method for electrical stimulation of salivation
A system, a device and a method for electrically detecting a lack of saliva in an oral cavity of an individual and for electrically stimulating the oral cavity so as to induce production of saliva from at least one salivary gland are disclosed. The system includes a control device for detecting a measure of salivation in the individual and for delivering electrical impulses to the oral cavity of the individual, a check device for checking a state of the control device and for modifying at least one parameter of the control device, and, a computer device for exchanging information with the check device.
Owner:SALIWIZER ITD
Polymeric stent useful for the treatment of the salivary gland ducts and method for using the same
Owner:NAHLEILI ODED
Device for simulating digestion in oral cavities and method for applying device
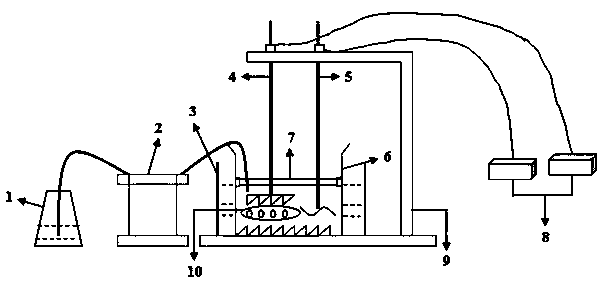
The invention discloses a device for simulating digestion in oral cavities and a method for applying the device. The device comprises components including a saliva dish, a constant-flow pump, a water bath kettle, a grinder, a stirrer, a reactor, an electric controller and the like. The method includes regulating the temperature of the water bath kettle until the temperature of the water bath kettle reaches 37 DEG C, adding saliva into the saliva dish, adding solution or dispersion liquid of to-be-tested substances into the reactor, starting the constant-flow pump and adding the saliva into the reactor; enabling the grinder and the stirrer to simulate chewing actions of teeth and stirring actions of tongues; continuously adding the saliva into the reactor via a hose to simulate a secretion effect of salivary glands; replacing water in the water bath kettle by boiling water to remove activity of salivary amylases after the secretion effect of the salivary glands is simulated for 30-40 minutes, repeatedly carrying out a digestion simulation procedure by three times, and detecting digestion conditions of the to-be-tested substances by a liquid-phase process. The device and the method have the advantages that specific digestion simulated in the oral cavities is described in detail for the first time, structures of various portions of the oral cavities and corresponding functions of the structures of the various portions of the oral cavities are simulated, accordingly, first-step digestion conditions of various active or nutrient substances can be observed after the various active or nutrient substances are fed into human bodies, and the device and the method can assist in exploring digestion conditions of the various active or nutrient substances after the various active or nutrient substances are fed into the human bodies.
Owner:NANCHANG UNIV
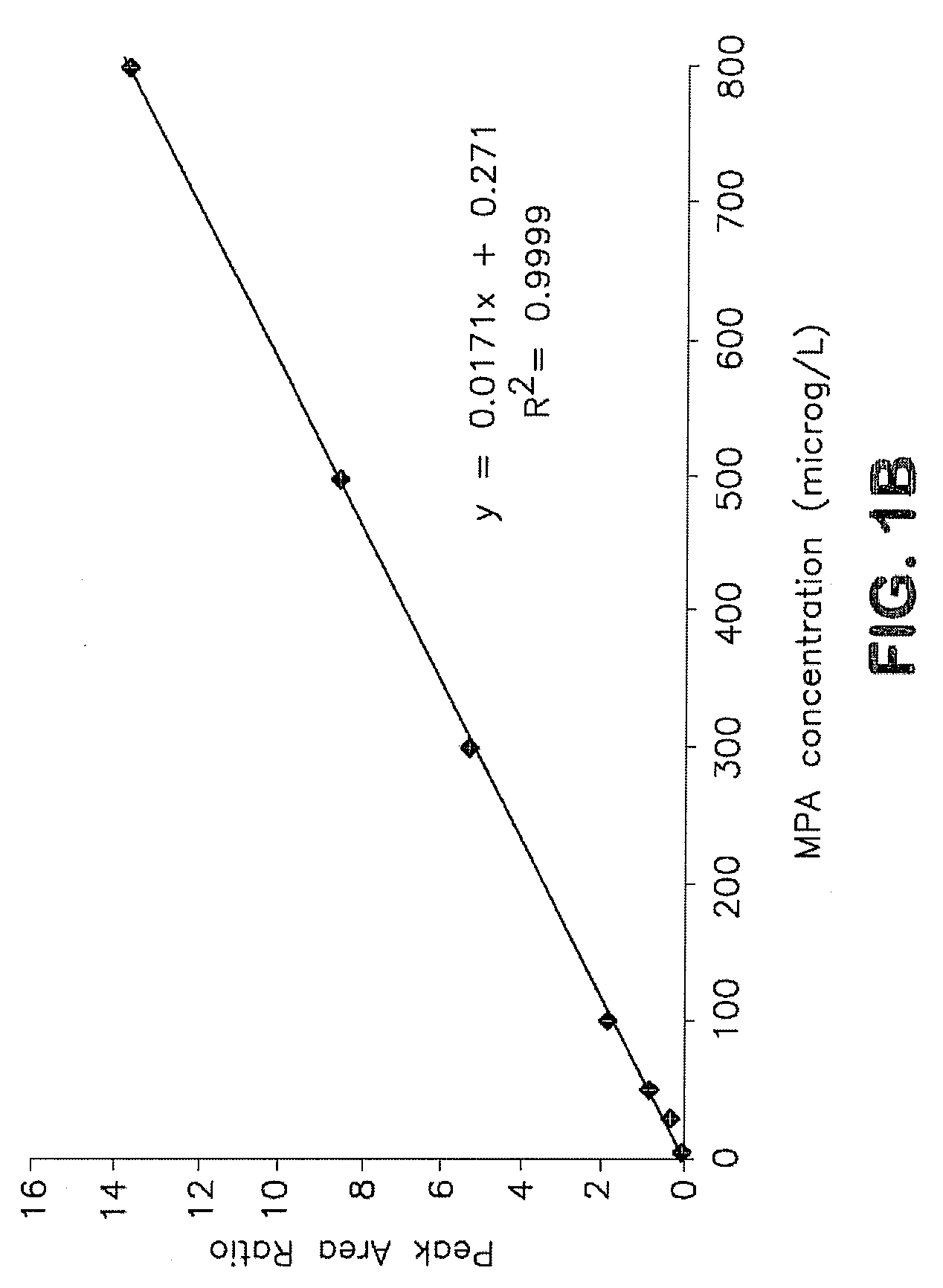
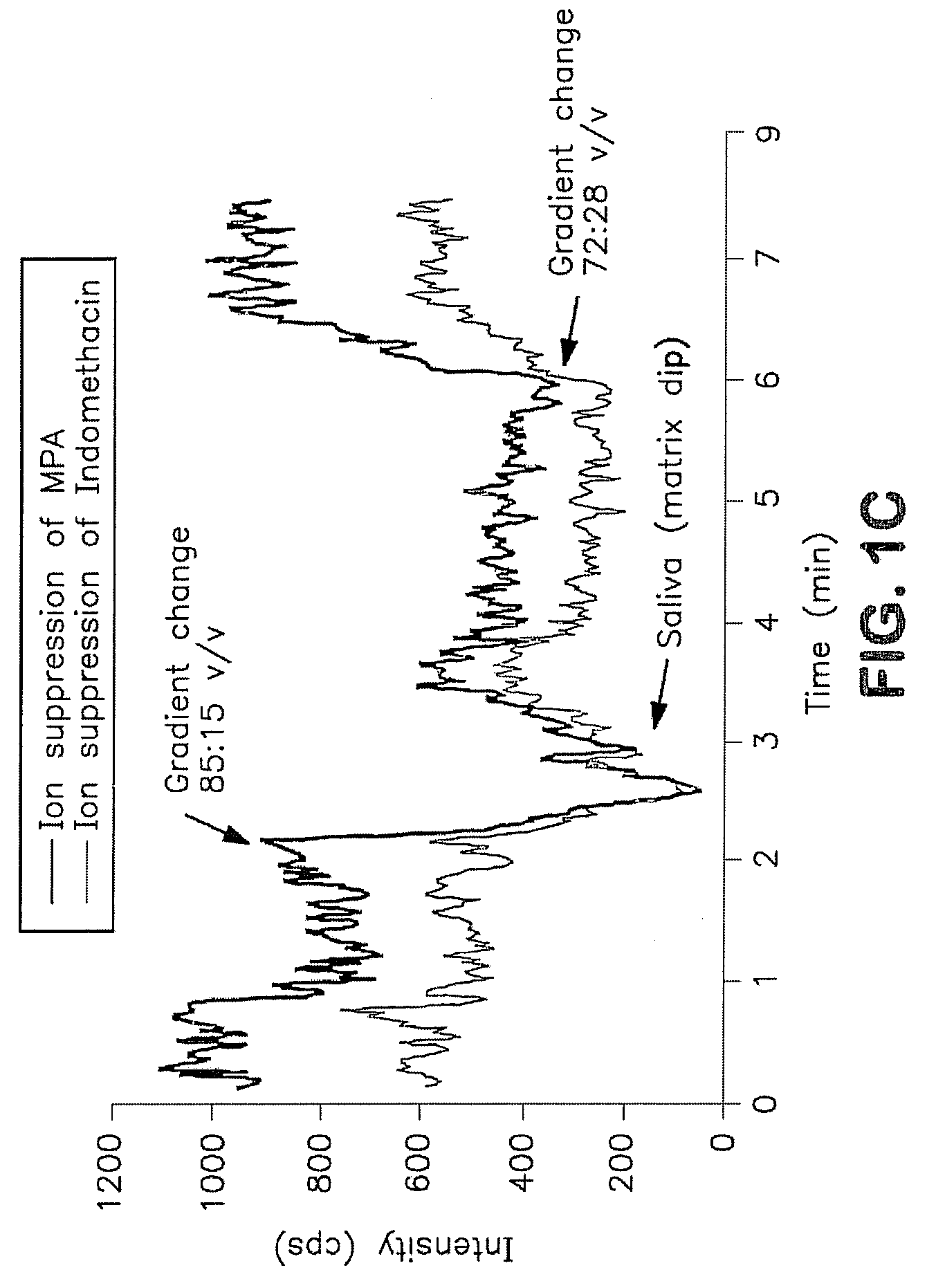
Analysis of mycophenolic acid in saliva using liquid chromatography tandem mass spectrometry
InactiveUS20080318322A1Component separationBiological testingSaliva sampleGas chromatography–mass spectrometry
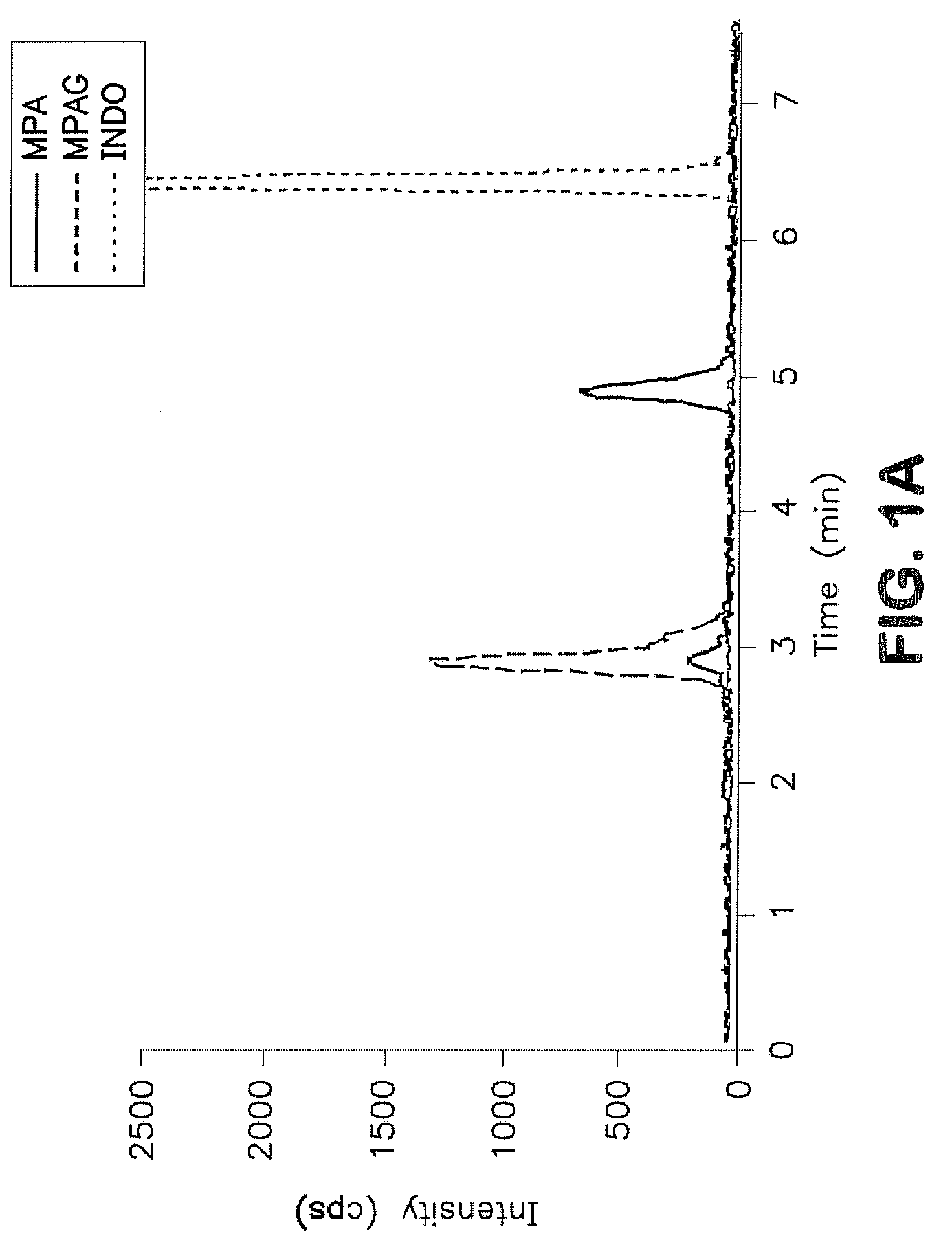
A method for mass spectrometric analysis of a saliva sample possibly containing mycophenolic acid or its metabolites mycophenolic acid phenyl glucuronide (MPAG) or mycophenolic acid acyl-glucuronide (Acyl-MPAG), including the steps: (a) providing a saliva sample containing one or more drug or metabolites; (b) deproteinating the sample; (c) separating the one or more drug or metabolites from the saliva sample; and (d) analyzing the one or more drug or metabolites using a mass spectrometer. The sample containing one or more MPA or metabolites is obtained from in an oral fluid based biological samples i.e. whole saliva or saliva obtained by chemical or mechanical stimulation or from specific salivary glands. The size of the sample contains one or more MPA or metabolites is at least about 100 microL. A kit for use in mass spectrometric analysis of a sample may contain one or more MPA or metabolites from saliva samples, comprising: (a) reagents for deproteinating of the saliva sample, including internal standards; (b) reagents for separating the one or more MPA or metabolites from the saliva sample; (c) reagents for analyzing the one or MPA or metabolites using a mass spectrometer; (d) a solution of one or more MPA or metabolites in saliva samples; and (e) instructions for analyzing the one or more MPA or saliva using a mass spectrometer. The kit includes (a) mobile phase solutions; (b) a chromatography column; and (c) a quality control specimen.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
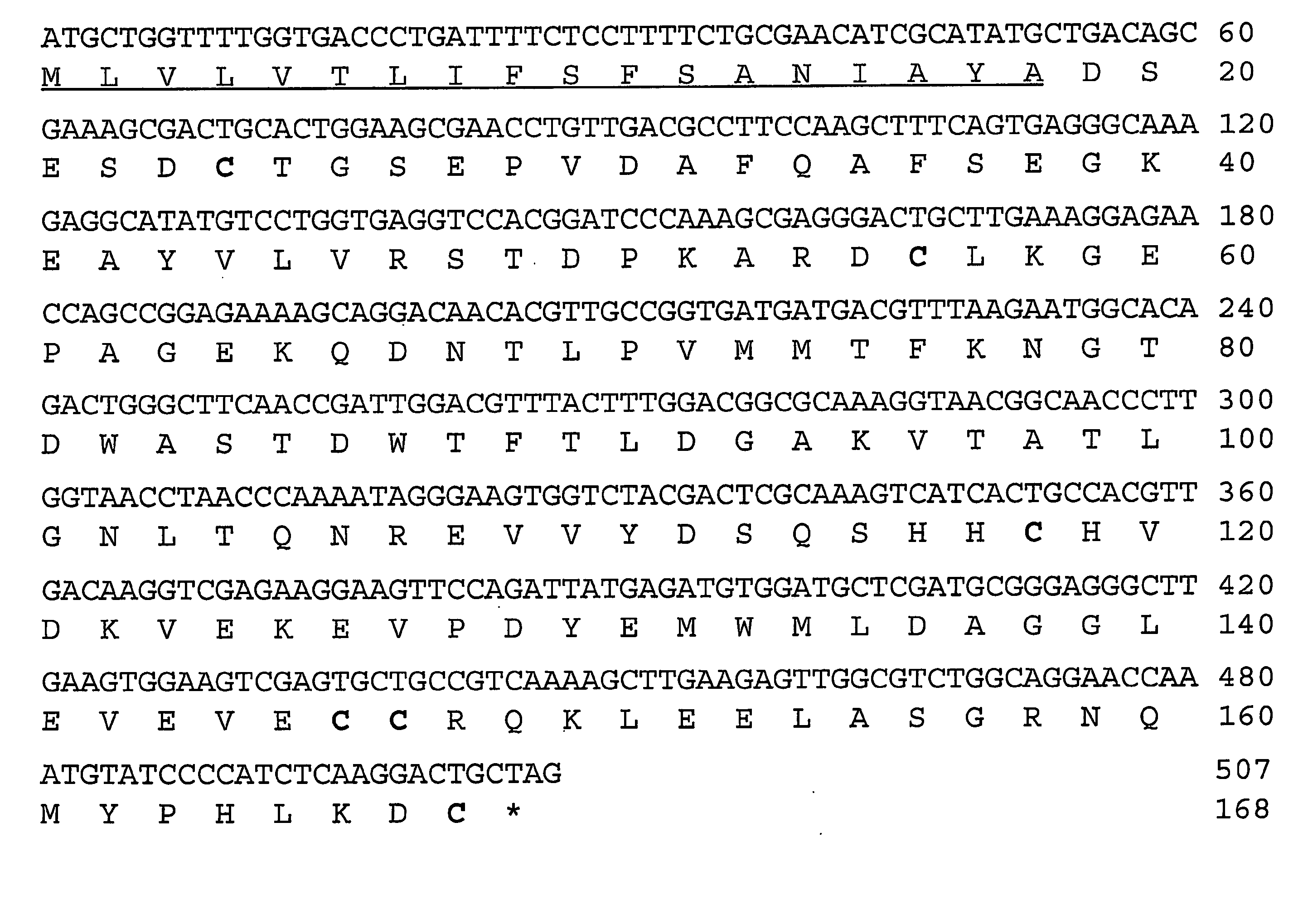
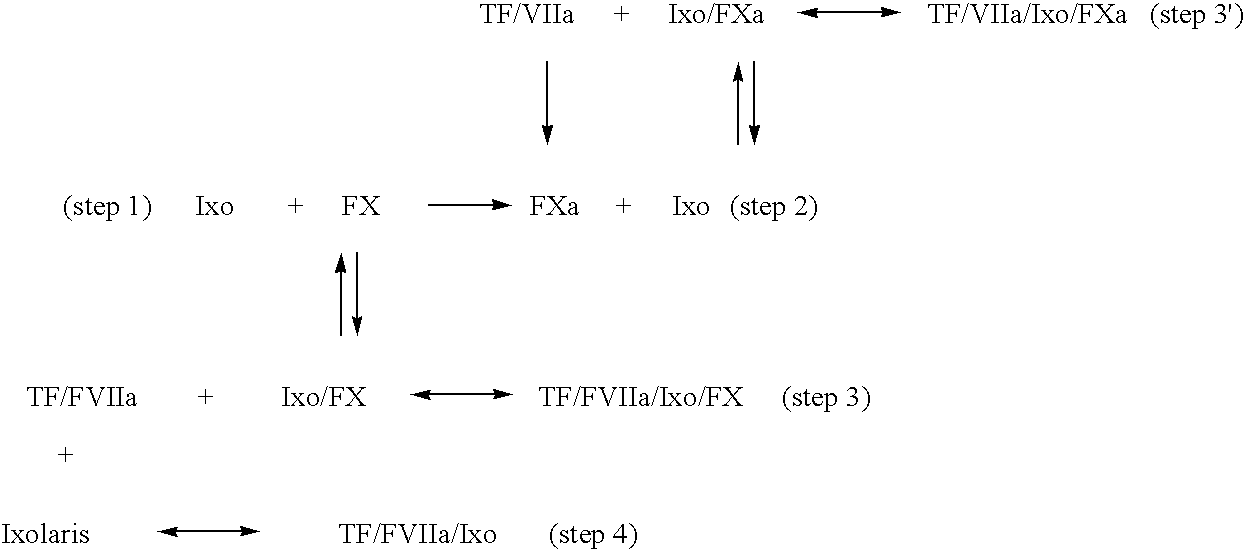
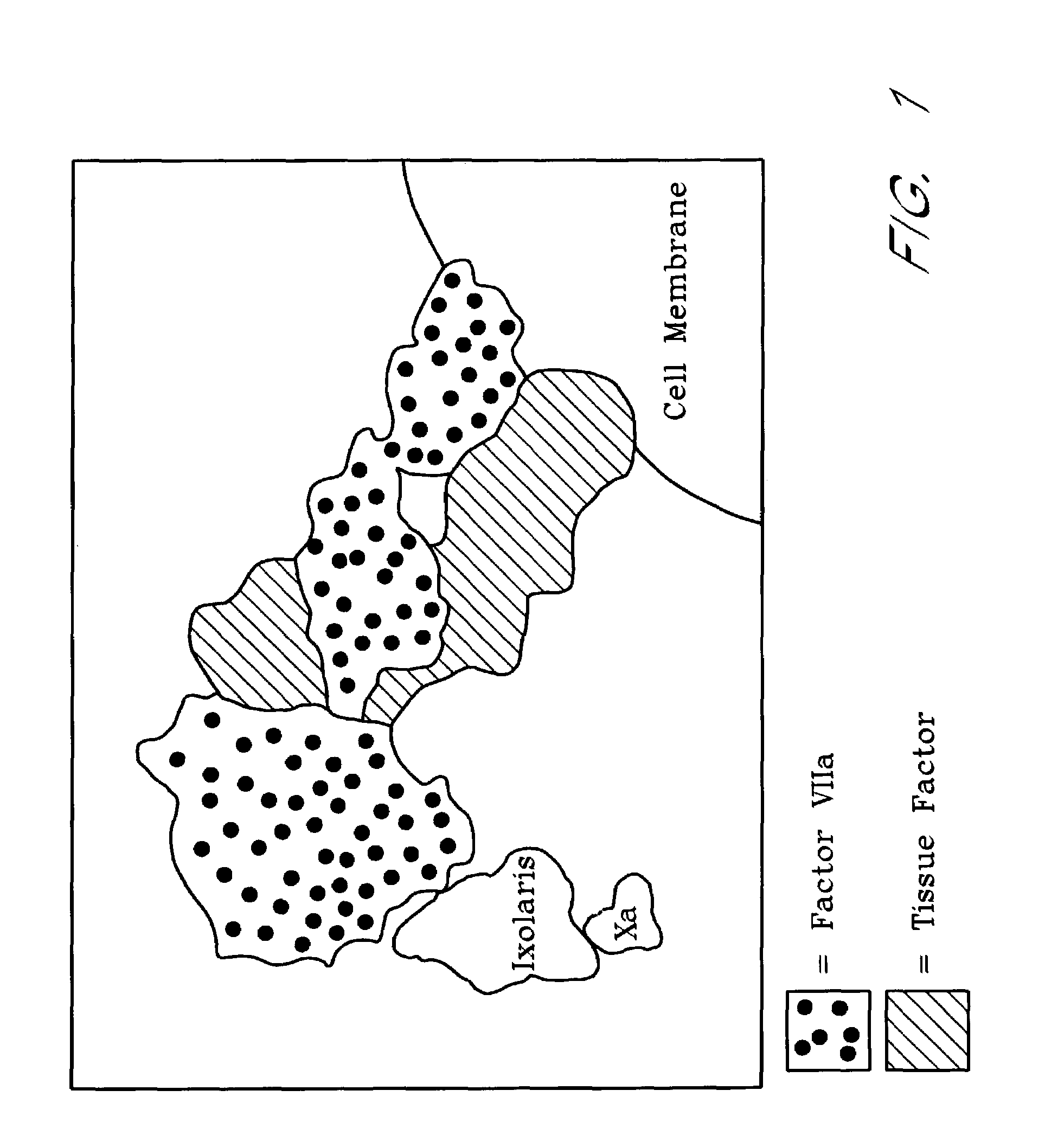
Ixodes scapularis tissue factor pathway inhibitor
InactiveUS7078508B2BacteriaPeptide/protein ingredientsTissue factor pathway inhibitorAnticoagulant activity
Ixolaris, a novel protein with anticoagulant activity is described. Ixolaris can be isolated from the salivary glands of ticks or made by recombinant methods using various DNA expression techniques.
Owner:HEALTH & HUMAN SERVICES DEPT OF THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Methods for eradication of nanobacteria
InactiveUS20050036904A1Preventing nanobacterial infectionInhibit and prevent growthOrganic active ingredientsBiocideDental pulp stonesCalcification
Nanobacteria contribute to pathological calcification in the human and animal body, including diseases such as kidney stones, salivary gland stones, dental pulp stones and atherosclerosis. The present invention provides methods for sterilizing articles contaminated with nanobacteria. The present invention also provides methods of treating patients infected with nanobacteria. In particular, the present invention provides a method for preventing the recurrence of kidney stones in a patient that has suffered from kidney stones, comprising administration of an antibiotic, a bisphosphonate, or a calcium chelator, either alone or in combination, in an amount effective to inhibit or prevent the growth and development of nanobacteria.
Owner:KAJANDER OLAVI E +1
Modeling method of Sjogren syndrome mouse model
InactiveCN106110315ALow physiological stateLow mental stateAntibody medical ingredientsMulti siteSjögren syndrome
The invention discloses a modeling method of an Sjogren syndrome mouse model. The modeling method comprises the following steps: killing mice, taking out bilateral salivary glands and peeling off capsules and connective tissues; washing with PBS (Poly Butylenes Succinate); adding the PBS according to the amount of adding 0.5ml of the PBS into each salivary gland; shearing the salivary glands into pieces, and uniformly homogenizing and centrifuging in an ice bath; then taking supernatant and quantifying salivary gland antigens by adopting a BCA (Bicinchoninic Acid) protein quantifying method; adjusting the concentration of the salivary gland antigens to be 4mg / ml by utilizing the PBS; adding equal quantity of an FCA (Freund Complete Adjuvant) or an FIA (Freund Incomplete Adjuvant) and diluting the concentration to be 2mg / ml; repeatedly blowing and beating until two liquid phases are dissolved mutually to form an ivory color; randomly grouping C57BL / 6 mice and shaving off furs on the backs of the mice; carrying out intradermal multi-site injection of 2mg / ml mouse salivary gland antigens prepared by the FCA on the back and tails of the mice on the current day and the 7th day, wherein the injection amount is 0.1ml per mouse; injecting equal quantity of the salivary gland antigens prepared by the FIA on the 14th day through the same method; after modeling for about 6 weeks, detecting indexes and screening the successfully modeled mice. The modeling method disclosed by the invention is high in modeling efficiency and short in modeling time.
Owner:魏伟
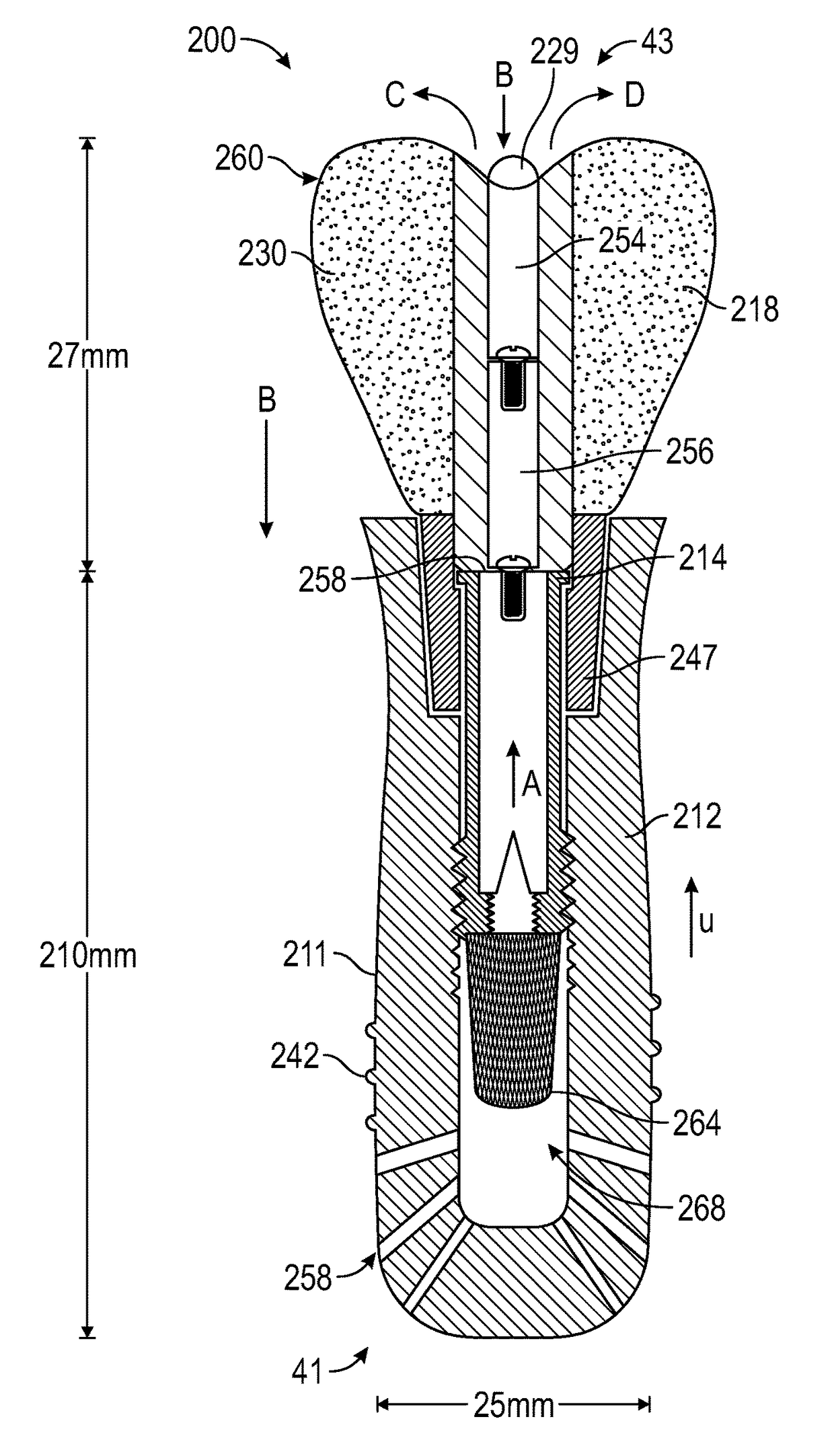
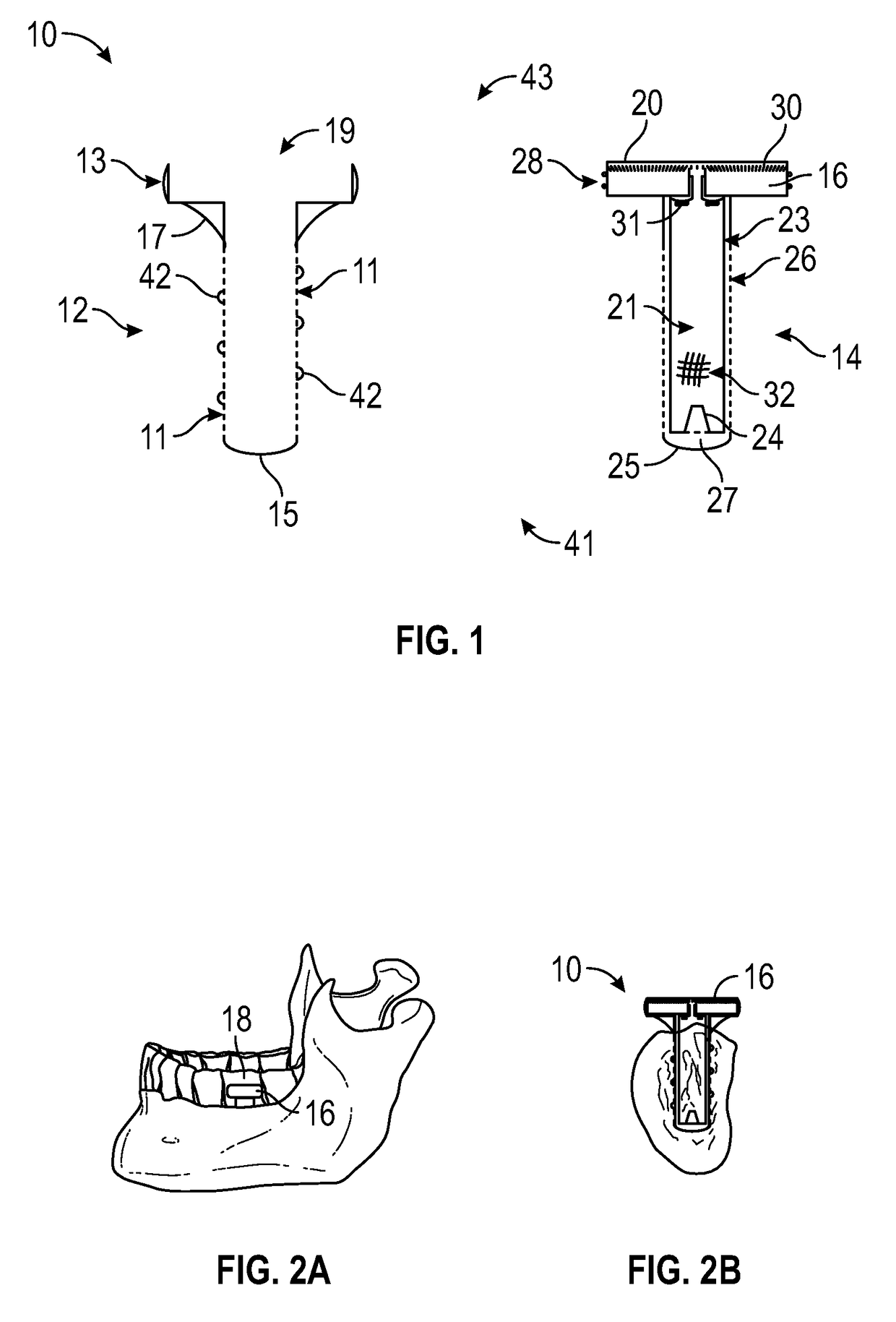
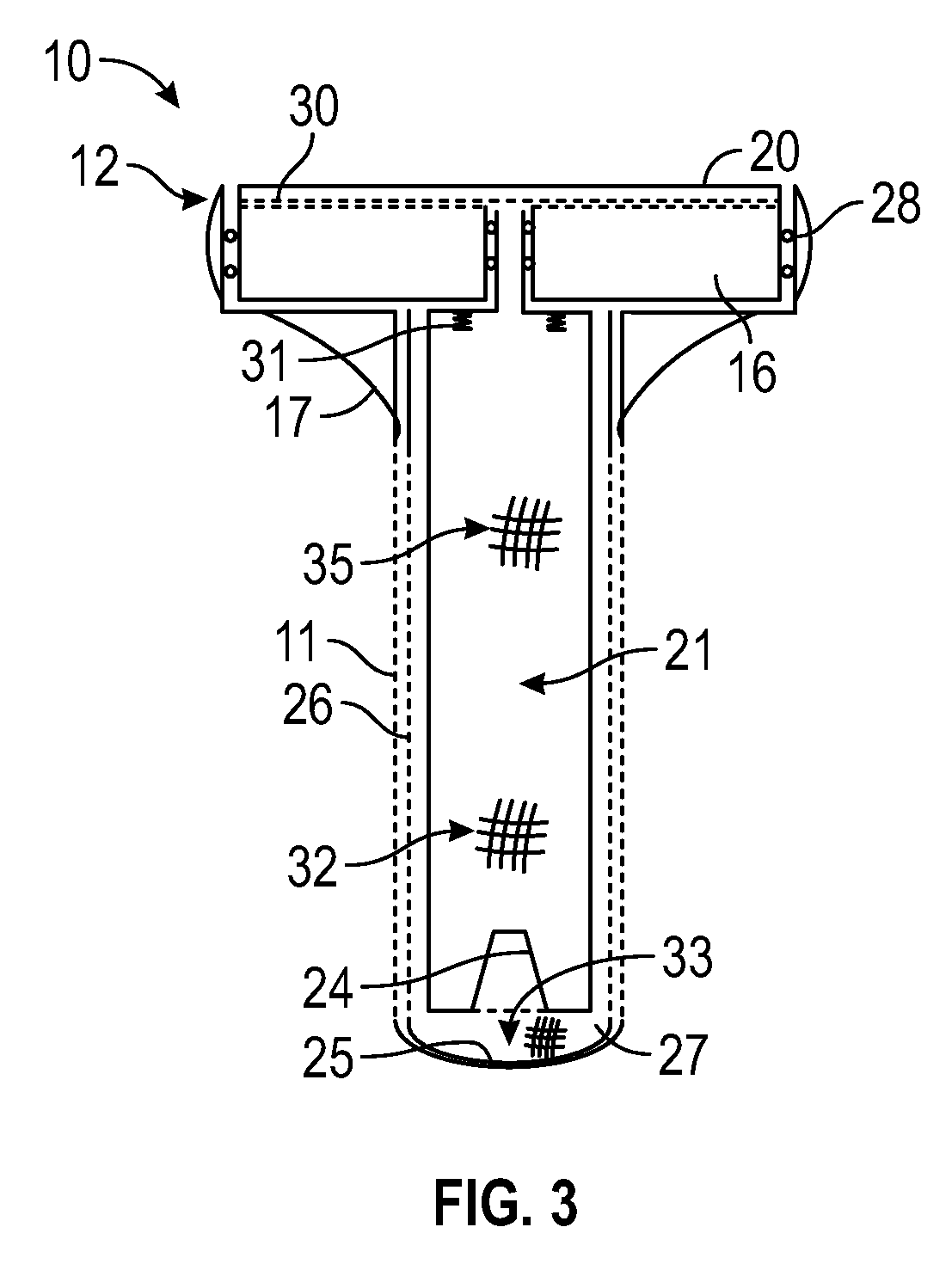
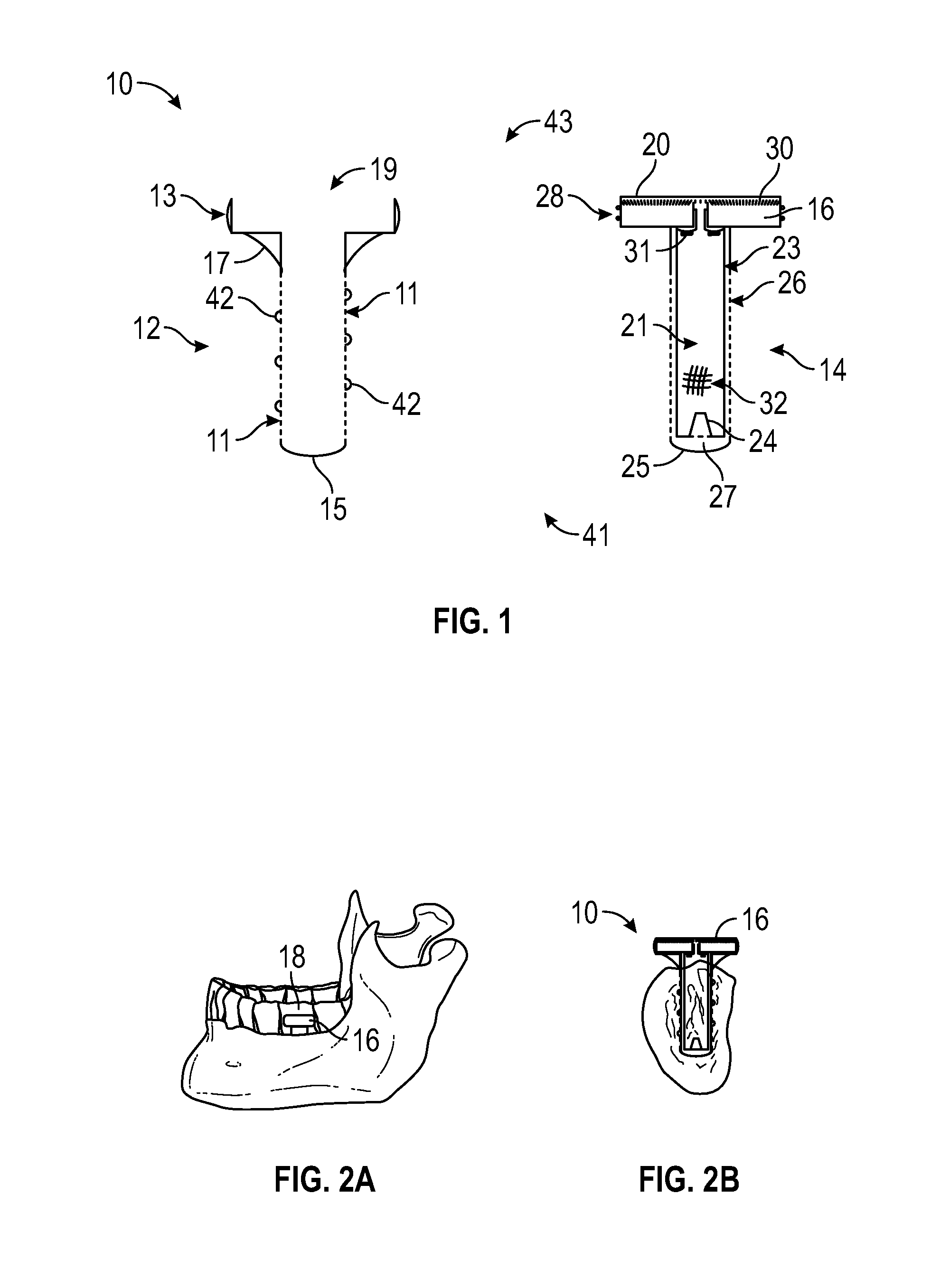
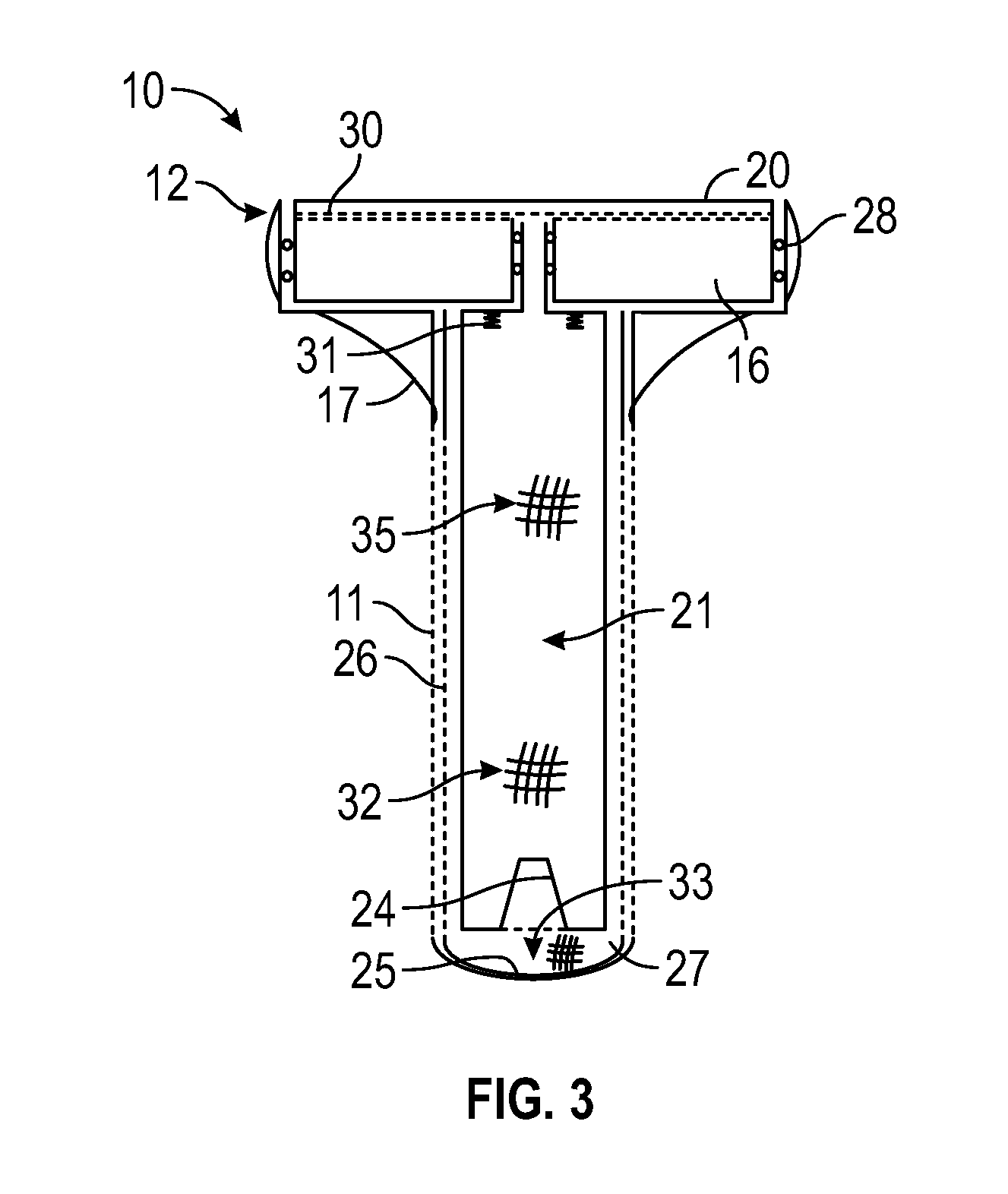
Artificial salivary gland
ActiveUS9872757B2Restore saliva flowConstant rateDental implantsTeeth fillingArtificial salivasIon exchange
Artificial salivary gland devices and assemblies are provided. The present disclosure provides artificial salivary pump / gland devices and assemblies, and related methods of use. One embodiment utilizes the interstitial / marrow fluid reservoir within the underlying mandibular or maxillary bone as a source for replacement saliva. The salivary pump / assembly, which is implantable in the mandibular or maxillary bone as a dental implant and driven by incidental tooth contact and masticatory forces, harvests interstitial / marrow fluid and treats it via semi-permeable membrane technology and soluble particles as a continuously available saliva replacement. Masticatory forces and tooth contact power the pump to both harvest interstitial / marrow fluid and drive flow through a bed of ion-exchange resins and / or soluble particles to adjust fluid chemistry providing a continuously available saliva-like solution. Exemplary devices and assemblies can also be utilized to introduce beneficial bacteria into the oral cavity and / or be utilized as a delivery system for drugs / therapeutic agents.
Owner:UNIV OF CONNECTICUT
Methods for the diagnosis and treatment of sjögren's syndrome
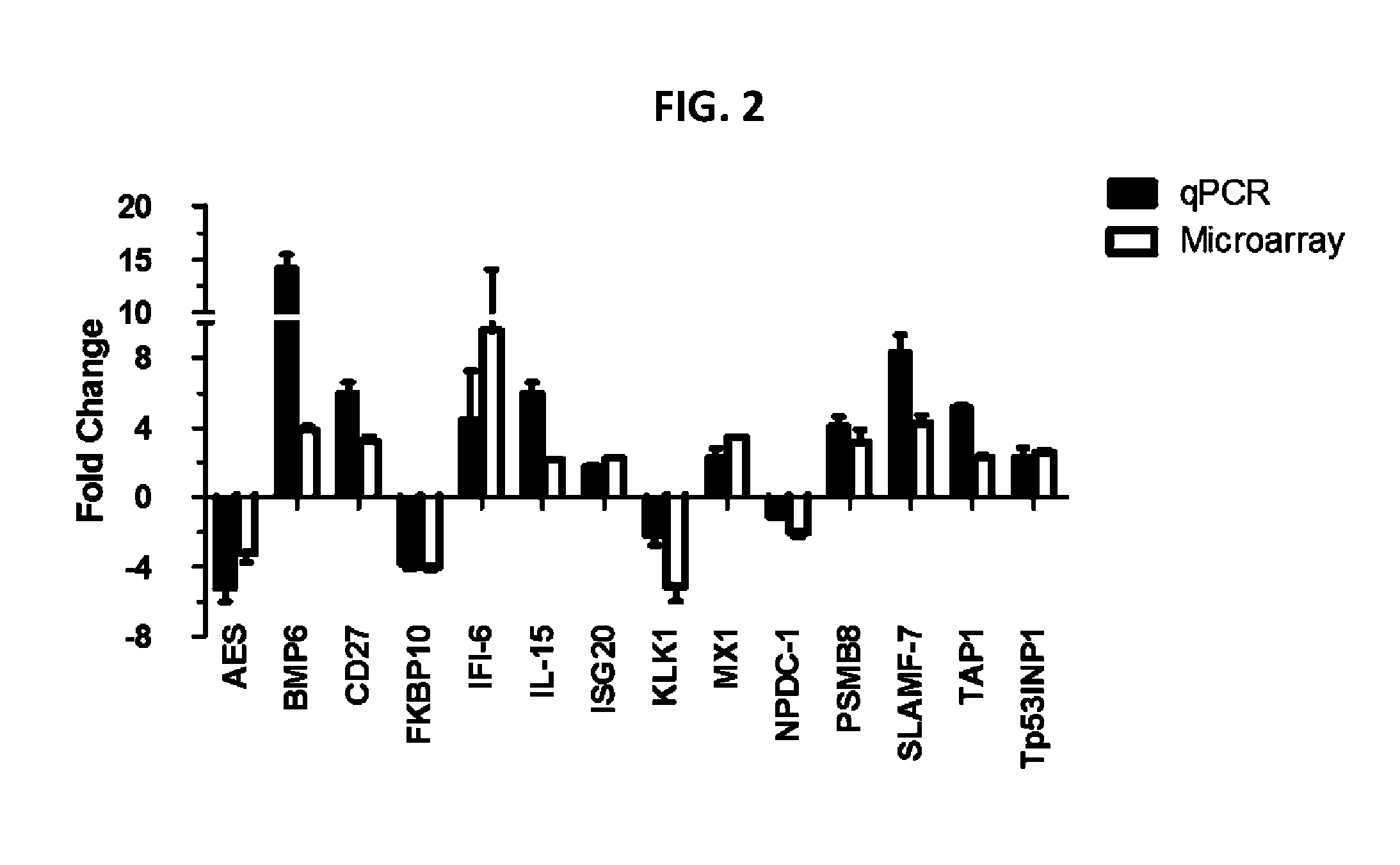
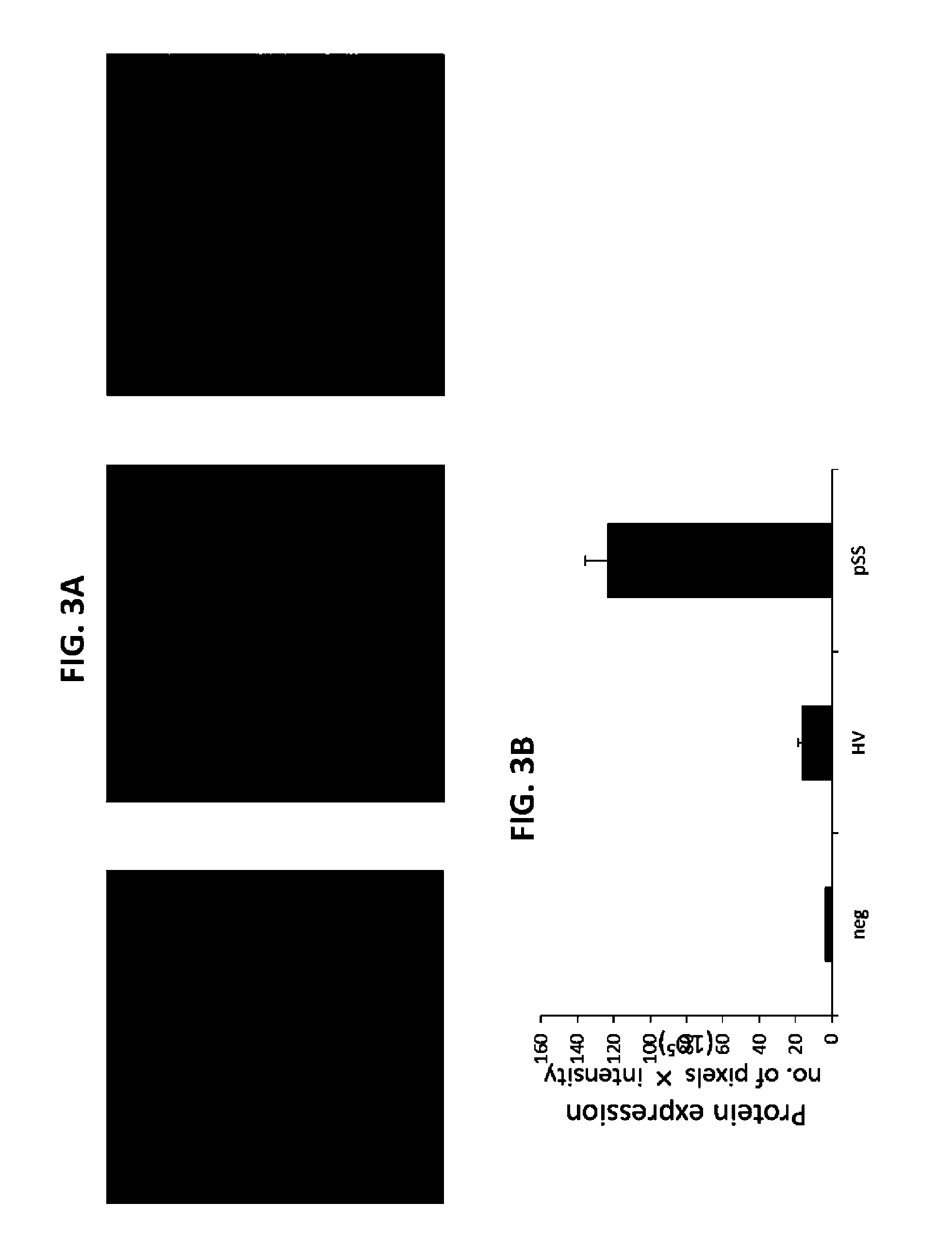
Described herein is the finding that patients with Sjögren's syndrome exhibit a statistically significant increase in expression of BMP6 in the salivary gland, relative to healthy control subjects. Also described herein is the finding that overexpression of BMP6 in the salivary glands of mice results in an increase in electrical potential across the salivary gland. Thus, provided herein are methods of diagnosing a subject as having Sjögren's syndrome, or at risk for developing Sjögren's syndrome, by measuring the level of BMP6 expression in a salivary gland of a subject, measuring electrical potential in a salivary gland of a subject, or both. Also provided herein are methods of treating a subject with Sjögren's syndrome by administering an agent that inhibits expression of BMP6 expression or activity. Also described herein is the use of XIST and MECP2 as diagnostic and therapeutic targets for male Sjögren's syndrome patients.
Owner:UNITED STATES OF AMERICA
Polycistron, vector for specific expression of polycistron through salivary glands and construction method thereof
ActiveCN106086068AReduce distractionsGuaranteed independenceNucleic acid vectorFermentationBiotechnologyPectinase
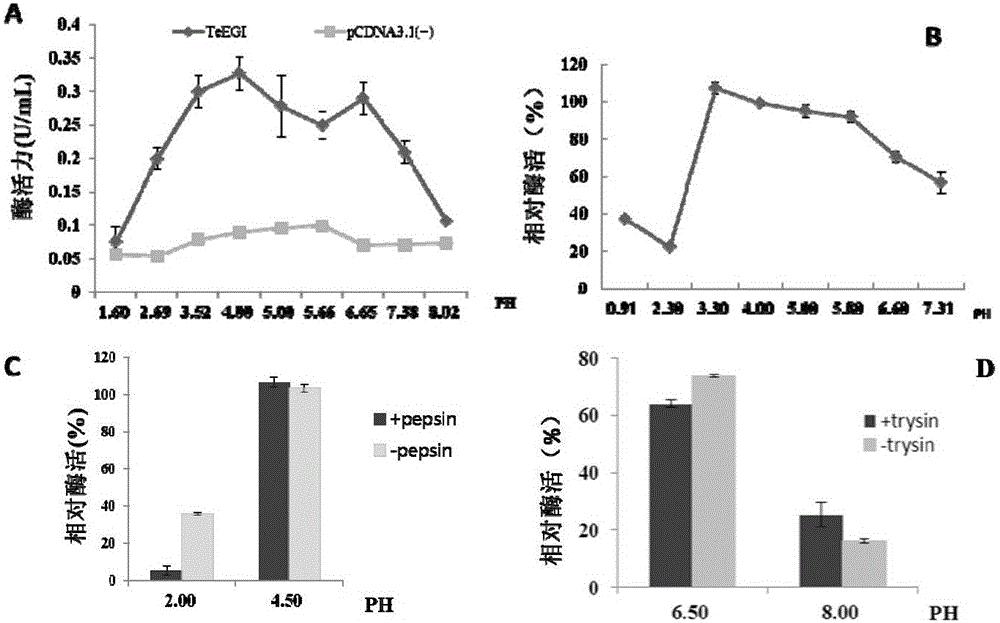
The invention discloses polycistron, a vector for specific expression of the polycistron through salivary glands and a construction method thereof. The multi gene co-expressed polycistron has a base sequence shown as SEQ ID No:1; the vector for specific expression of the polycistron through salivary glands has a nucleotide sequence shown as SEQ ID No:3, and is obtained by constructing with an eukaryotic expression vector of the polycistron, namely pCD-PXAT, and an pPB-mPSP-neoGFP vector as raw materials. The constructed polycistron by the invention can co-express cellulase, xylanase, dextranase, pectase and phytase, includes all the major members of the non-starch polysaccharide enzyme (NSP Enzyme) and phytase in the conventional feed, these enzymes are well compatible with the pH environment of the gastrointestinal tract in animals, have good pepsin tolerance and trypsin tolerance, and always maintain high activity, the defects and problems of the traditional feed which uses an enzyme preparation in the processing, production and use process are overcome, and because of animal's own secretion, there is almost no cost problem.
Owner:WENS FOOD GRP CO LTD +1
Pharmaceutical composition using gonadotropin - releasing hormone (GNRH) combined variants as immunogen
InactiveUS20110250196A1Faster and more potent immunological responseVigorous immunocastration actionPeptide/protein ingredientsDigestive systemHuman tumorHuman fertility
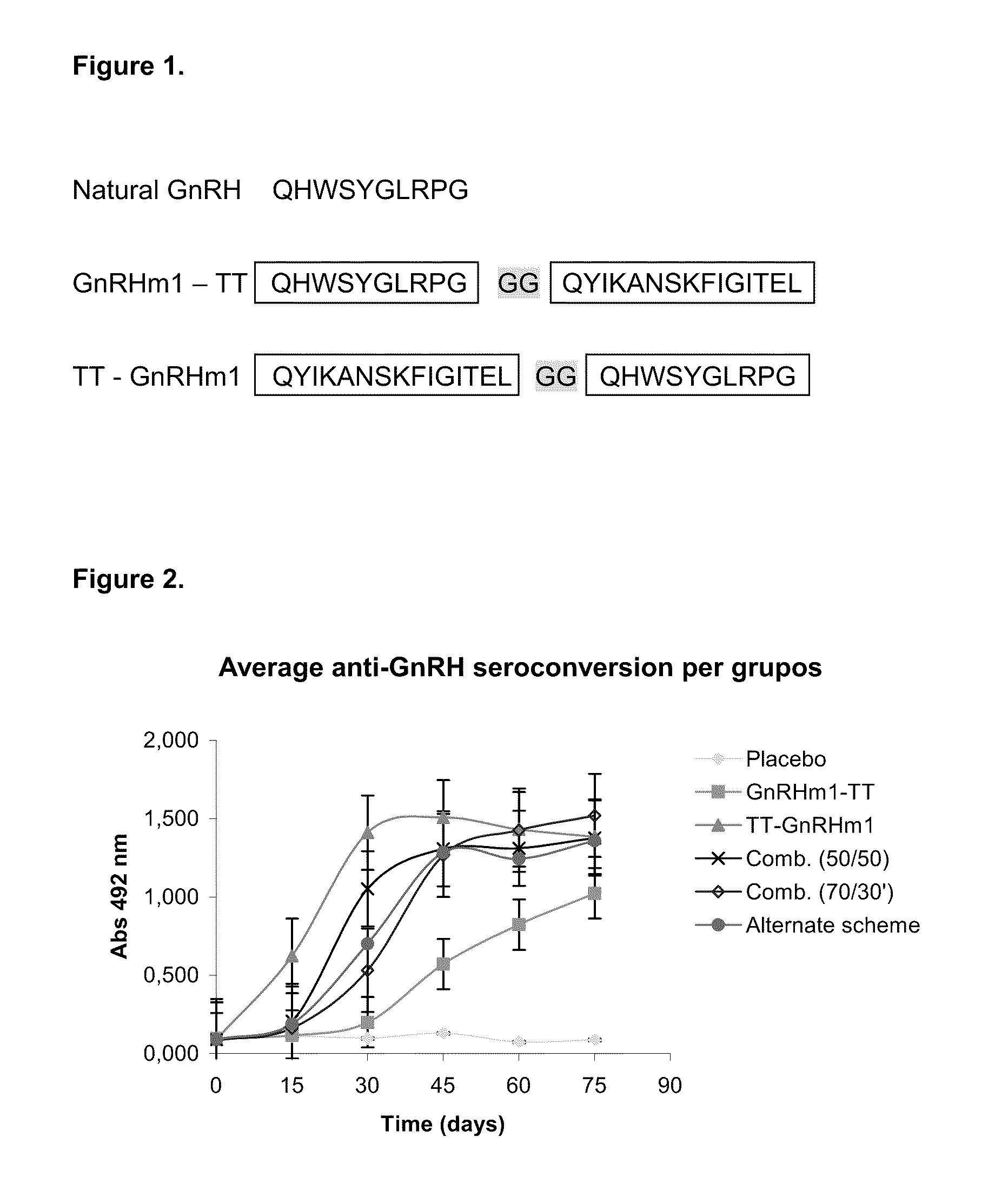
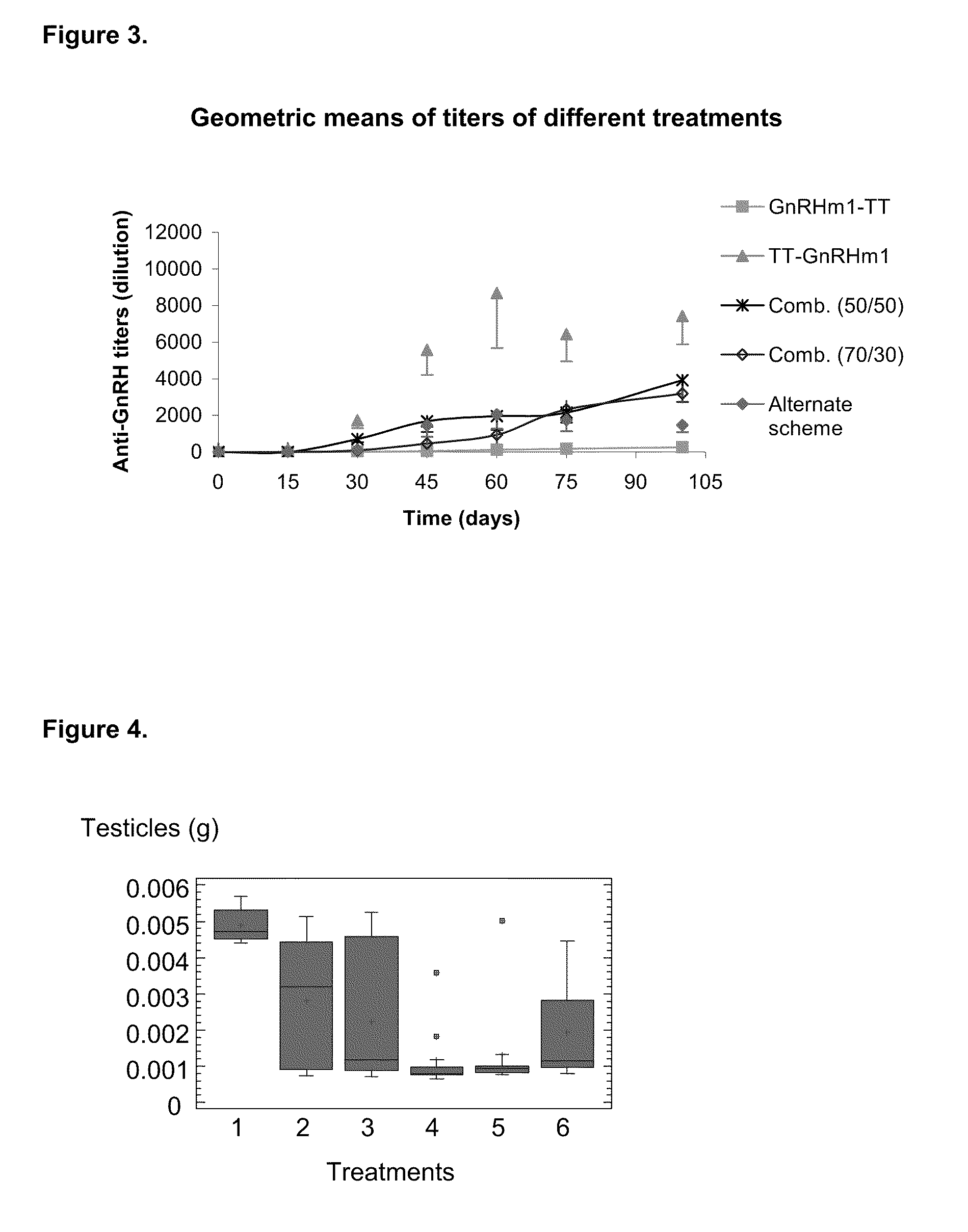
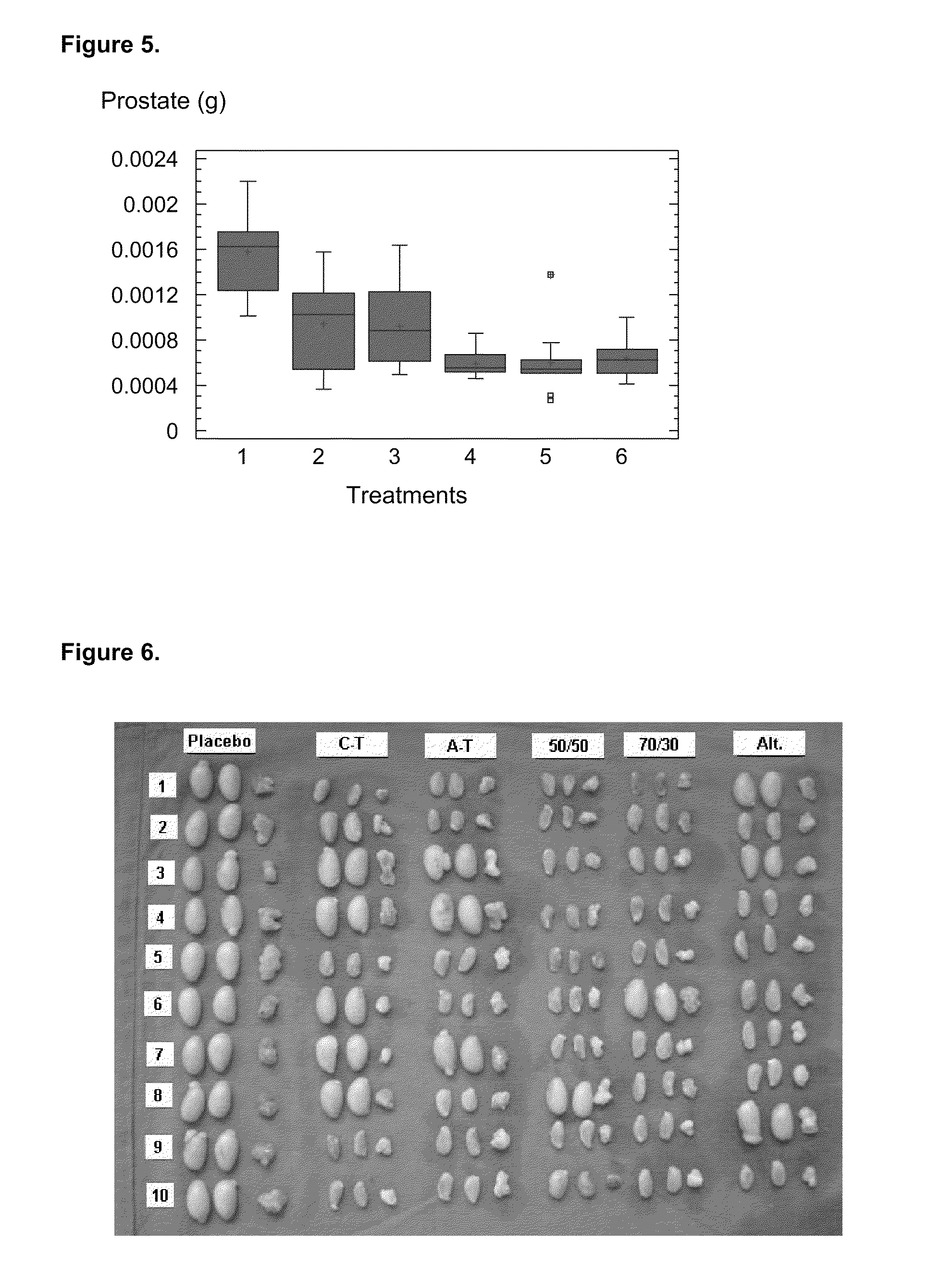
A pharmaceutical composition using natural gonadotropin-releasing hormone (GnRH), and / or some of its mimetic peptides, indistinctly bound by its amino or carboxyl extremes to a carrier molecule; in one case by its carboxyl extreme and in the other case by the amino terminal extreme, thus eliciting a faster and more potent immunological response against the endogenous GnRH hormone. This finally leads to the ablation of the GnRH and consequently of the rest of the involved hormones in the stream GnRH / LH-FSH / Testosterone-(estrogens). An advantage of this formulation consists on facilitating the exposition to the immune system of a greater number of epitopes of the GnRH or its mimetics, minimizing thus the steric hindrance produced by the carriers. This invention has a direct application in the castration of pets and animals of economic interest, in the control of human fertility as well as in the treatment of hormone-sensitive tumors, such as that of the prostate, the breast, ovary, the endometry, testicles, hypophysis, salivary glands and other kinds of human tumors.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Carbohydrate based cellulase inhibitors as feeding stimulants in termites
InactiveUS20080107619A1Increase feed rateIncrease termite mortalityBiocideAnimal repellantsEndoglucanase activityDigestion
A method, composition and system for controlling termites wherein single carbohydrate-based compounds are used as both cellulase inhibitors and feeding stimulants. Di-saccharides, cellobioimidazole (CBI), fluoro-methyl cellobiose (FMCB), and mono-saccharides, fluoro-methyl glucose (FMG) and analogs thereof inhibit termite cellulose digestion, which leads to starvation or stimulates termite feeding to cause mortality. CBI, FMCB and FMG were tested against enzyme fractions that represented endogenous (foregut / salivary gland / midgut) and symbiotic (hindgut) termite cellulases in vitro and in vivo. Feeding stimulation by di-saccharides results in greater cellulase inhibitor intake throughout midrange concentrations (1 mM-10 mM), which is associated with significant termite mortality. In contrast, the monosaccharide inhibitor, FMG did not stimulate feeding, but did inhibit feeding at concentrations above 1 mM, causing mortality. With modification to create longer β-glycosidic chain lengths, the cellulase inhibitors identified herein can also be targeted to endoglucanase activity for increased efficacy and use as novel termite control compositions.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Artificial Salivary Gland
ActiveUS20160278909A1Restore saliva flowConstant rateDental implantsTeeth fillingArtificial salivasIon-exchange resin
Artificial salivary gland devices and assemblies are provided. The present disclosure provides artificial salivary pump / gland devices and assemblies, and related methods of use. One embodiment utilizes the interstitial / marrow fluid reservoir within the underlying mandibular or maxillary bone as a source for replacement saliva. The salivary pump / assembly, which is implantable in the mandibular or maxillary bone as a dental implant and driven by incidental tooth contact and masticatory forces, harvests interstitial / marrow fluid and treats it via semi-permeable membrane technology and soluble particles as a continuously available saliva replacement. Masticatory forces and tooth contact power the pump to both harvest interstitial / marrow fluid and drive flow through a bed of ion-exchange resins and / or soluble particles to adjust fluid chemistry providing a continuously available saliva-like solution. Exemplary devices and assemblies can also be utilized to introduce beneficial bacteria into the oral cavity and / or be utilized as a delivery system for drugs / therapeutic agents.
Owner:UNIV OF CONNECTICUT
Balsam pear lozenge and method for preparing the same
A balsam pear buccal tablet takes balsam pear powder as the major ingredient and American ginseng saponin and mannite as accessories; wherein the weight percent of the ingredients is: 50-55 percent of balsam pear powder, 25-30 percent of American ginseng saponin and residual amount of mannite. The preparation comprises the following steps: 1) selecting high-quality fresh balsam pear as the raw material, dehydrating the fresh balsam pear by low temperature technology and preparing into balsam pear powder; 2) uniformly mixing the powder material based on the weight percent of the ingredients; 3) pressing the powder into tablets by the conventional method, packing or filling hermetically. The balsam pear buccal tablet has the advantage that, in the balsam pear buccal tablet prepared by the method, the hypoglycemic peptide in the main raw material--balsam pear, has the efficacy of reducing blood sugar safely and stably, improving the function of pancreas, increasing the activity of insulin cells of human body, improving the capability of regulating blood sugar, regulating blood fat and lowering blood pressure. Furthermore, the bitter principle in the balsam pear can stimulate the secretion of salivary gland to increase, better relive the symptoms of dry mouth and thirst of diabetics. The balsam pear buccal tablet is easy to eat, which is a pure natural healthcare product.
Owner:沃德(天津)营养保健品有限公司
An apyrase encoding gene of Aedes albopictus, and preparation method and application of protein thereof
The invention falls into the field of biotechnology, and relates to an apyrase encoding gene of Aedes albopictus, and preparation method and application of protein thereof. In the invention, RNA is extracted from the salivary gland of Aedes albopictus; the apyrase encoding gene of Aedes albopictus is obtained by PCR; and an apyrase expression vector pET19b-apyrase is constructed. After abundant expression, purification and further identification, it has been proved that, the recombinant apyrase of Aedes albopictus has equal immune activity as natural apyrase of Aedes albopictus, can cause immune response of human, and has inhibitory activity against platelet aggregation. The inventive apyrase of Aedes albopictus can be used in the diagnosis of anaphylactic diseases induced by Aedes albopictus, and also be used as antithrombotic for treating thrombus caused by platelet aggregation.
Owner:FUDAN UNIV
Immunotoxins directed against c-erbB-2(HER-2/neu) related surface antigens
Novel immunotoxins and methods of treating neoplastic diseases are provided. More specifically, immunotoxins comprised conjugation of a c-erbB-2 targeting moiety and a cell growth modulator are provided. These immunotoxins specifically and selectively kill tumor cells that over-express the c-erbB-2 protein. The novel immunotoxins would be useful in treating human mammary carcinomas, human ovarian carcinomas, lung carcinomas, gastric tumors, salivary gland adenocarcinomas, and colon adenocarcinomas.
Owner:RES DEVMENT FOUND
Yao horsefly salivary gland immunoregulation peptide, gene and application thereof
ActiveCN101220091ASimple structureEasy to synthesizeImmunological disordersFermentationTyrosineAntibacterial activity
The invention relates to a salivary gland immunoregulation polypeptide of a dieu cleg and the related genes and the application, belonging to the biomedical field. The immunoregulation polypeptide is a linear polypeptide coded with the Chinese Tabanidae salivary gland immunoregulation polypeptide genes, the sequence of which is glycin- glycin- valine- serine- glycin- valine- serine- aspartic acid- phenylalanine- glutamate- praline- isoleucine- glutamate- valine- serine- glycin- glutamate- aspartic acid- tyrosine- aspartic acid- serine- aspartic acid- glutamate- alanine- aspartic acid- glutamate- aspartic acid- glycin- lysine- alanine. The immunoregulation polypeptide gene is composed of 362 Nucleotides, wherein, the coding mature code salivary gland immunoregulation polypeptide of the dieu cleg is the 115th to the 204th nucleotide. The dieu cleg salivary gland immunoregulation polypeptide is used for inhibiting a plurality of cytokines, which is characterized by simple structure, convenient artificial synthesis and strong antibacterial activity, etc.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
A Chinese medicine preparation for easing-off head and neck neoplasm radiotheraphy secondary reaction
InactiveCN101161264AAvoid damagePromote repairDigestive systemRespiratory disorderWhite blood cellWhite blood cell number
The present invention relates to a traditional Chinese medicinal preparation (heat-clearing, blood-cooling and body fluid-promoting beverage) for relieving head or neck tumor radiation therapy side effect: mainly comprising stomatitis, neck skin reaction, hematology variation (mainly indicating leucocyte decreasing), pharynx and esophagus mucosa injury and salivary gland change and etc. It is composed of heat-clearing and antitoxic, blood-cooling and stasis-eliminating, qi-invigorating and body fluid-promoting, spleen and stomach conciliating traditional Chinese medicine, with monarch,minister,assistant and guide to process reasonable compatibility, its medicine constitution according to their weight ratio is: scutellaria ( fried with alcohol) 10 to 15 gram, honeysuckle 10 to 15 gram, forgythia fruit 10 to 15 gram, rehmanniae radix 13 to 18 gram, red sage root 10 to 15 percent, radix paeoniae rubrathe 10 to 15 gram, Chinese angelica 8 to 10 gram, ginseng 6 to 10 gram, Schisandra chinensis 5 to 8 gram, atractylodes rhizome 8 to 10 gram, raw glycyrrhiza 8 to 12 gram. By clinical experiment, effect is obvious. It has characteristics for reducing injury of radiation ray to around normal tissue, promoting around normal tissue repairing, relieving pain caused by radiotherapy side reaction, ensuring radiotherapy successful accomplishment.
Owner:曹锋
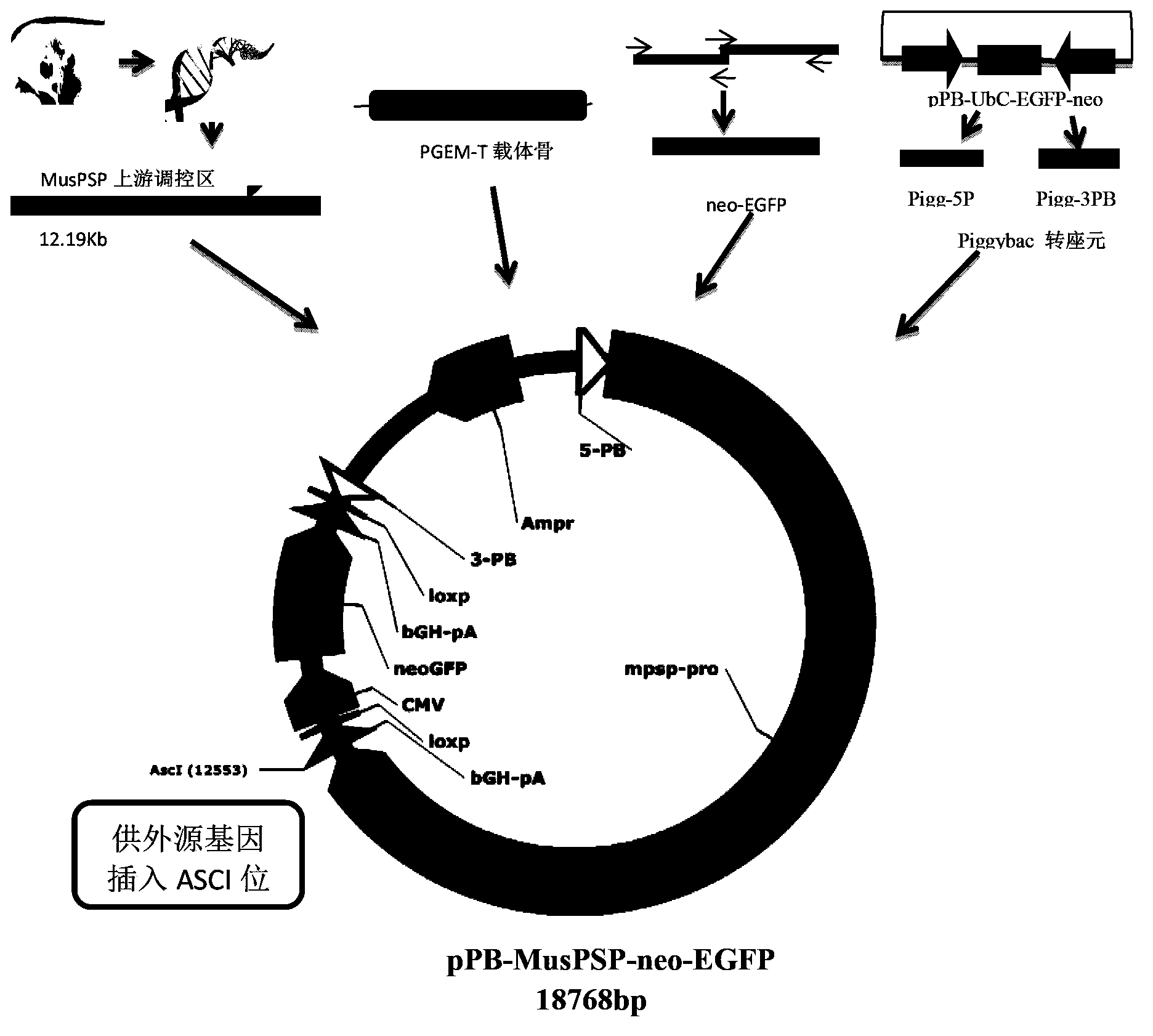
Specific transgenic carrier and construction method thereof
ActiveCN103509812AEasy to identifyIdentify directlyVector-based foreign material introductionPiggyBac Transposon SystemEnzyme system
The invention discloses a salivary gland tissue specific transgenic carrier and a construction method thereof. The salivary gland tissue specific transgenic carrier is obtained by emerging an upstream regulation region sequence of a mice parotid gland protein promoter and a visual screened neo-EGFP combined double-label to a piggyBac transposon system and a Lox-Cre enzyme system; the transgenic carrier can make protein express in a particular tissue to achieve a high-efficient large-section emerging efficiency. The carrier also has a green luminescence label which is convenient for screening and a neomycin resistance gene which is convenient for eukaryotic cell screening, and makes the screening of transgenic cells and identification of transgenic animals be simpler, more direct, and more accurate. The transgenic carrier provided by the invention is a salivary gland specific expression high-efficient integration universal carrier, and is capable of being applied to fields of transgenic animals such as transgenic mice and pig, and the like. The invention clones the upstream regulation region of FVB mice for the first time, the cloning method is simple and high efficient, and the difficulty in construction of a large section carrier is effectively overcome.
Owner:国科润风(广州)生物医药有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com