An apyrase encoding gene of Aedes albopictus, and preparation method and application of protein thereof
Aedes albopictus and protein technology, applied in botany equipment and methods, biochemical equipment and methods, genetic engineering, etc., can solve the difference in titer and specific antigen level, it is difficult to standardize and quantify, and limit the application of diagnosis and treatment, etc. problems, to achieve high output and constant production conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of Aedes albopictus salivary gland
[0037] Adult (1-5 days old) non-blood-sucking Aedes albopictus, select female mosquitoes, remove wings, pick salivary glands under a dissecting microscope, wash with PBS, and store at -80°C for later use.
Embodiment 2
[0038] Example 2 Acquisition of Aedes albopictus Apyrase Encoding Gene
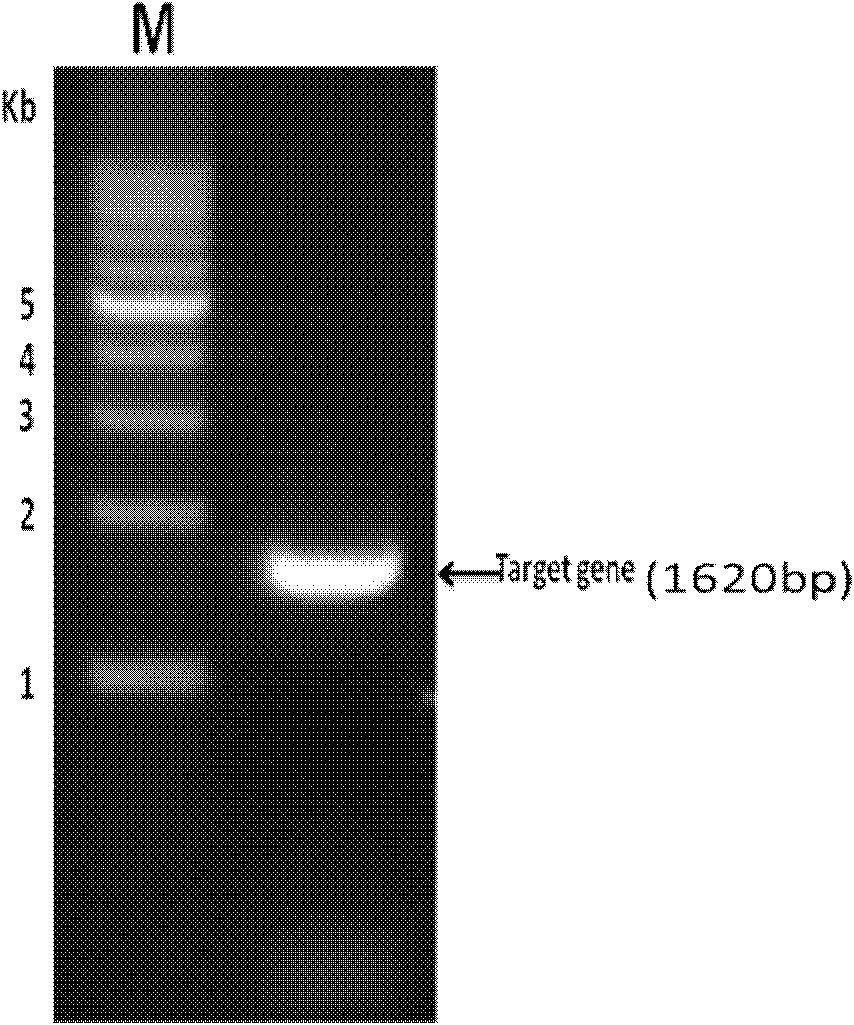
[0039] Using the RNeasy Plus minikit, according to the instructions of the kit, total RNA of Aedes albopictus was extracted and stored at -70°C for future use. RT-PCR was performed using ProtoScriptTM First Strand cDNA Synthesis kit to obtain cDNA. Using APY-Nde I-S 5'-CCCATATGATGGATAATATGCCCGCTGATAAAGATG-3', APY-XhoI-AS 5'-CCCTCGAGTCAAGTACATGGTGAACCTGCTTTACAT-3' as template, PCR amplified the gene fragment encoding Apyrase, and the PCR product was detected by 0.8% agarose gel electrophoresis. Its size is 1620bp (such as figure 1 shown).
Embodiment 3
[0040] Example 3 Construction of recombinant expression plasmid pET19b-Apyrase
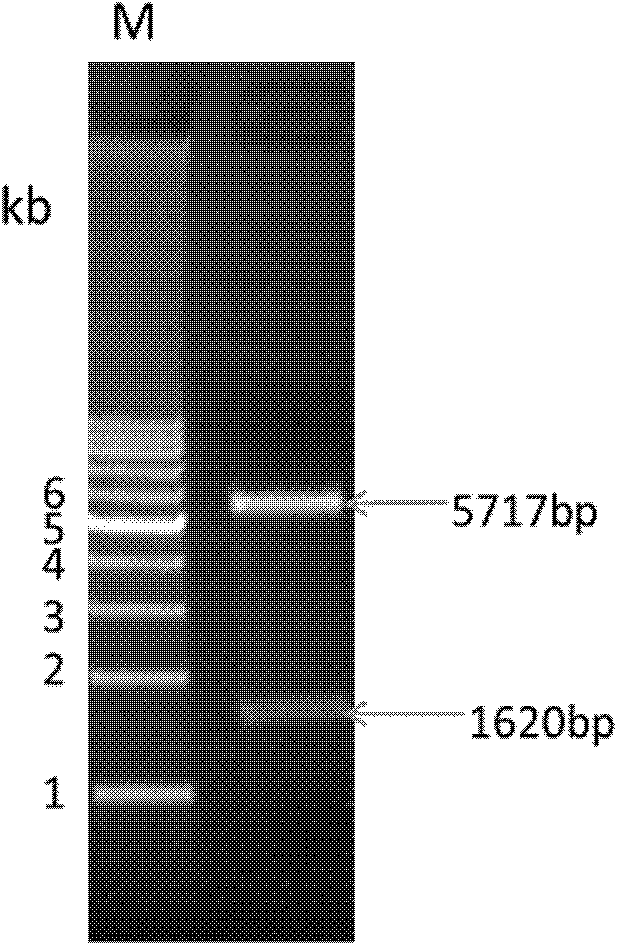
[0041] The PCR amplification product was purified with QIAquick PCR Purification Kit, and then the PCR amplification product was digested with NdeI and XhoI and inserted into the pET19b vector. Transform into Escherichia coli JM109, spread the bacterial solution on LB agar plates (containing ampicillin 100 μg / ml, chloramphenicol 34 μg / ml), culture overnight at 37°C, pick clones, extract recombinant plasmids for double enzyme digestion identification , the results showed that the target band was consistent with the size of the PCR product of the Apyrase gene fragment, indicating that the recombinant plasmid pET19b-Apyrase of Aedes albopictus Apyrase (such as figure 2Shown) The recombinant plasmid was sequenced and identified by Shanghai Handsome Biotechnology Co., Ltd., and the amino acid sequence was deduced using GENETYX-MAC Ver.11.0 software, and compared with AAC37218.1 (Aedes aegypti apyrase)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com